Abstract
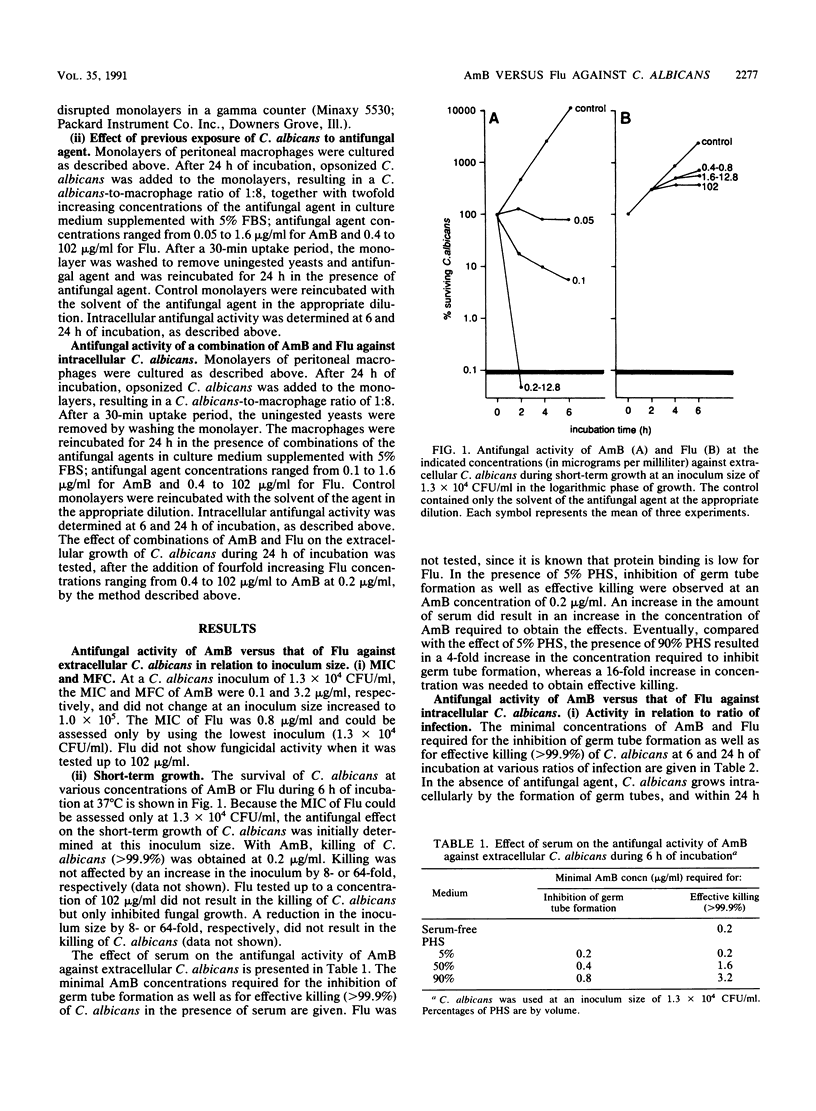
The effects of amphotericin B and fluconazole on the extracellular and intracellular growth of Candida albicans were studied. With respect to the extracellular growth of C. albicans, antifungal activity was measured in terms of MICs and minimal fungicidal concentrations as well as by determination of the concentration that effectively killed (greater than 99.9%) C. albicans in the absence or presence (amphotericin B only) of serum. Amphotericin B was highly active in terms of killing, even at an increased inoculum size. In the presence of serum, amphotericin B activity was substantially reduced. For fluconazole, activity was restricted to inhibition of fungal growth, even after the inoculum size was reduced. With respect to the intracellular growth of C. albicans, antifungal activity was measured by using monolayers of murine peritoneal macrophages infected with C. albicans and was measured in terms of inhibition of germ tube formation as well as effective killing (greater than 99%) of C. albicans. Amphotericin B was highly active against C. albicans. At an increased ratio of infection, amphotericin B activity was slightly reduced. Fluconazole had no antifungal activity. Neither a reduction in the ratio of infection nor exposure of C. albicans to fluconazole prior to macrophage ingestion resulted in activity against intracellular C. albicans by fluconazole. Previous exposure of C. albicans to amphotericin B resulted in increased intracellular activity of amphotericin B. The intracellular antifungal activity of the combination of fluconazole with amphotericin B was less than that of amphotericin B alone. Amphotericin B showed fungicidal activity against C. albicans growing both extracellularly and intracellularly, whereas fluconazole inhibited growth only of extracellular C. albicans. A slight antagonistic effect between fluconazole and amphotericin B was found with respect to intracellular as well as extracellular C. albicans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brammer K. W., Farrow P. R., Faulkner J. K. Pharmacokinetics and tissue penetration of fluconazole in humans. Rev Infect Dis. 1990 Mar-Apr;12 (Suppl 3):S318–S326. doi: 10.1093/clinids/12.supplement_3.s318. [DOI] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Candidacidal mechanisms of peritoneal macrophages activated with lymphokines or gamma-interferon. J Med Microbiol. 1989 Mar;28(3):173–181. doi: 10.1099/00222615-28-3-173. [DOI] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes W. E. Azole antifungal drugs: old and new. Ann Intern Med. 1988 Aug 1;109(3):177–179. doi: 10.7326/0003-4819-109-3-177. [DOI] [PubMed] [Google Scholar]
- Drutz D. J. In vitro antifungal susceptibility testing and measurement of levels of antifungal agents in body fluids. Rev Infect Dis. 1987 Mar-Apr;9(2):392–397. doi: 10.1093/clinids/9.2.392. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Shadomy S. In vitro and in vivo evaluation of antifungal agents. Eur J Clin Microbiol Infect Dis. 1989 Apr;8(4):352–361. doi: 10.1007/BF01963469. [DOI] [PubMed] [Google Scholar]
- Evron R. In vitro phagocytosis of Candida albicans by peritoneal mouse macrophages. Infect Immun. 1980 Jun;28(3):963–971. doi: 10.1128/iai.28.3.963-971.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. A., Lee P. G., Tarry W. F. Fluconazole (UK-49,858) treatment of candidiasis in normal and diabetic rats. Antimicrob Agents Chemother. 1989 Jul;33(7):1042–1045. doi: 10.1128/aac.33.7.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. A., Shen S. H., Haddad J., Tarry W. F. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 1989 Sep;33(9):1443–1446. doi: 10.1128/aac.33.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev. 1988 Apr;1(2):187–217. doi: 10.1128/cmr.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N. Antifungal susceptibility tests. Antimicrob Agents Chemother. 1987 Dec;31(12):1867–1870. doi: 10.1128/aac.31.12.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N. Susceptibility of Candida albicans and other yeasts to fluconazole: relation between in vitro and in vivo studies. Rev Infect Dis. 1990 Mar-Apr;12 (Suppl 3):S272–S275. doi: 10.1093/clinids/12.supplement_3.s272. [DOI] [PubMed] [Google Scholar]
- Gallis H. A., Drew R. H., Pickard W. W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990 Mar-Apr;12(2):308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- Grant S. M., Clissold S. P. Fluconazole. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycoses. Drugs. 1990 Jun;39(6):877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- Horn R., Wong B., Kiehn T. E., Armstrong D. Fungemia in a cancer hospital: changing frequency, earlier onset, and results of therapy. Rev Infect Dis. 1985 Sep-Oct;7(5):646–655. doi: 10.1093/clinids/7.5.646. [DOI] [PubMed] [Google Scholar]
- Hughes C. E., Beggs W. H. Action of fluconazole (UK-49,858) in relation to other systemic antifungal azoles. J Antimicrob Chemother. 1987 Feb;19(2):171–174. doi: 10.1093/jac/19.2.171. [DOI] [PubMed] [Google Scholar]
- Humphrey M. J., Jevons S., Tarbit M. H. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother. 1985 Nov;28(5):648–653. doi: 10.1128/aac.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi G. S., Spitzer E. D. Testing of organisms for susceptibility to triazoles: is it justified? Eur J Clin Microbiol Infect Dis. 1989 May;8(5):387–389. doi: 10.1007/BF01964051. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Medoff G., Kobayashi G. S. Effects of amphotericin B on macrophages and their precursor cells. Antimicrob Agents Chemother. 1977 Jan;11(1):154–160. doi: 10.1128/aac.11.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. T., Mehta K., Lopez-Berestein G., Juliano R. L. Effect of liposomal amphotericin B on murine macrophages and lymphocytes. Infect Immun. 1985 Feb;47(2):429–433. doi: 10.1128/iai.47.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier F. Candidiasis. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):438–447. doi: 10.1007/BF01964058. [DOI] [PubMed] [Google Scholar]
- Meunier F., Lambert C., Van der Auwera P. In-vitro activity of SCH39304 in comparison with amphotericin B and fluconazole. J Antimicrob Chemother. 1990 Feb;25(2):227–236. doi: 10.1093/jac/25.2.227. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Cutler J. E. In vitro studies of the interaction of murine phagocytic cells with Candida albicans. J Reticuloendothel Soc. 1981 Jan;29(1):23–34. [PubMed] [Google Scholar]
- Nugent K. M., Couchot K. R. Effects of sublethal concentrations of amphotericin B on Candida albicans. J Infect Dis. 1986 Oct;154(4):665–669. doi: 10.1093/infdis/154.4.665. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Couchot K. R., Gray L. D. Effect of Candida morphology on amphotericin B susceptibility. Antimicrob Agents Chemother. 1987 Feb;31(2):335–336. doi: 10.1128/aac.31.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. Relative inhibition factors--a novel approach to the assessment of antifungal antibiotics in vitro. J Antimicrob Chemother. 1984 Jan;13(1):31–43. doi: 10.1093/jac/13.1.31. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Cheesman S. L., Abbott A. B. Antifungal effects of fluconazole (UK 49858), a new triazole antifungal, in vitro. J Antimicrob Chemother. 1986 Oct;18(4):473–478. doi: 10.1093/jac/18.4.473. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Savani D. V., Durack D. T. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrob Agents Chemother. 1986 Apr;29(4):579–583. doi: 10.1128/aac.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M. A., Rogers T. R. Interactions in vitro between polyenes and imidazoles against yeasts. J Antimicrob Chemother. 1991 Apr;27(4):491–506. doi: 10.1093/jac/27.4.491. [DOI] [PubMed] [Google Scholar]
- Richardson K., Brammer K. W., Marriott M. S., Troke P. F. Activity of UK-49,858, a bis-triazole derivative, against experimental infections with Candida albicans and Trichophyton mentagrophytes. Antimicrob Agents Chemother. 1985 May;27(5):832–835. doi: 10.1128/aac.27.5.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. E., Galgiani J. N. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother. 1986 Sep;30(3):418–422. doi: 10.1128/aac.30.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Dismukes W. E. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother. 1988 Jan;32(1):1–8. doi: 10.1128/aac.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D. A. The new generation of antifungal drugs. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):732–735. doi: 10.1007/BF01975038. [DOI] [PubMed] [Google Scholar]
- Troke P. F., Andrews R. J., Brammer K. W., Marriott M. S., Richardson K. Efficacy of UK-49,858 (fluconazole) against Candida albicans experimental infections in mice. Antimicrob Agents Chemother. 1985 Dec;28(6):815–818. doi: 10.1128/aac.28.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troke P. F., Andrews R. J., Pye G. W., Richardson K. Fluconazole and other azoles: translation of in vitro activity to in vivo and clinical efficacy. Rev Infect Dis. 1990 Mar-Apr;12 (Suppl 3):S276–S280. doi: 10.1093/clinids/12.supplement_3.s276. [DOI] [PubMed] [Google Scholar]
- Van t Wout J. W., Mattie H., van Furth R. Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against a systemic Candida albicans infection in normal and neutropenic mice. Antimicrob Agents Chemother. 1989 Feb;33(2):147–151. doi: 10.1128/aac.33.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. J., Aoki S., Mechinaud F., Bacher J., Lee J., Rubin M., Pizzo P. A. Effects of preventive, early, and late antifungal chemotherapy with fluconazole in different granulocytopenic models of experimental disseminated candidiasis. J Infect Dis. 1990 Apr;161(4):755–760. doi: 10.1093/infdis/161.4.755. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Lee J., Aoki S., Mechinaud F., Bacher J., Lecciones J., Thomas V., Rubin M., Pizzo P. A. Experimental basis for use of fluconazole for preventive or early treatment of disseminated candidiasis in granulocytopenic hosts. Rev Infect Dis. 1990 Mar-Apr;12 (Suppl 3):S307–S317. doi: 10.1093/clinids/12.supplement_3.s307. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Pizzo A. Treatment of systemic fungal infections: recent progress and current problems. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):460–475. doi: 10.1007/BF01962595. [DOI] [PubMed] [Google Scholar]
- Wildfeuer A., Laufen H., Haferkamp O. Interaction of fluconazole and human phagocytic cells. Uptake of the antifungal agent and its effects on the survival of ingested fungi in phagocytes. Arzneimittelforschung. 1990 Sep;40(9):1044–1047. [PubMed] [Google Scholar]



