Abstract
Emerging evidence suggests that myocyte enhancer factor 2 (MEF2) transcription factors act as effectors of neurogenesis in the brain, with MEF2C the predominant isoform in developing cerebrocortex. Here, we show that conditional knockout of Mef2c in nestin-expressing neural stem/progenitor cells (NSCs) impaired neuronal differentiation in vivo, resulting in aberrant compaction and smaller somal size. NSC proliferation and survival were not affected. Conditional null mice surviving to adulthood manifested more immature electrophysiological network properties and severe behavioral deficits reminiscent of Rett syndrome, an autism-related disorder. Our data support a crucial role for MEF2C in programming early neuronal differentiation and proper distribution within the layers of the neocortex.
Keywords: neurogenesis, synaptogenesis, autism, Rett syndrome
Knockdown of the transcription factor MEF2C in mature cerebrocortical neurons results in increased synaptic number and activity (1). To facilitate analysis of MEF2C function in early neuronal development, we engineered a conditional knockout in NSCs by crossing floxed Mef2c mice with Nestin-Cre mice. In contrast to the findings in more mature neurons, we found a striking alteration in the distribution of new neurons in the neocortex and the opposite effect on synaptic activity, i.e., decreased neurotransmission persisting into adulthood.
MEF2C belongs to the myocyte enhancer factor 2 (MEF2) subfamily of the MADS (MCM1-agamous-deficiens-serum response factor) gene family (2, 3). We cloned MEF2C from developing mouse brain, and Eric Olson and colleagues then discovered it in the heart (2, 4, 5). In cerebrocortex, MEF2 transcriptional activity is restricted to differentiated cortical neurons in a specific laminar pattern, and its distribution increases along the rostrocaudal axis (2, 4, 6). These features led to speculation on the potential role of MEF2C in the architechtonics of the cerebral cortex (2). Previous studies demonstrated an important role for MEF2C in heart development (7). In the CNS, MEF2C is involved in neuronal apoptosis (8) and synapse formation (1, 9) in vitro or in brain slices. Most recently, our laboratory discovered that a constitutively active form of MEF2C induces embryonic stem cells to commit to a neuronal fate in a virtually exclusive fashion (10). However, studies on the effect of endogenous MEF2C on CNS neurons in vivo were impeded by the embryonic lethality of conventional Mef2c-null mice because of cardiovascular defects at embryonic day (E) 9.5, before brain development (7). Here, we report that conditionally knocking out the Mef2c gene in neural progenitors causes abnormal aggregation and compaction of neurons migrating into the lower layers of the neocortex during development. Knockout mice surviving to adulthood manifest smaller, apparently less mature neurons and smaller whole brain size, with resultant aberrant electrophysiology and behavior.
Results
MEF2C Conditional Knockout Mice.
Knockout of the Mef2c gene is embryonic lethal because of severe heart developmental defects (7). Therefore, to investigate the role of MEF2C in brain development, we conditionally knocked out Mef2c in neural stem/progenitor cells by E9.5 by using a nestin-driven Cre [see supporting information (SI) Materials and Methods) (11). Heterozygous n-Cre+/Mef2cloxp/+ mice were indistinguishable from wild type (WT) and served as littermate controls. Evidence for Cre-mediated recombination included PCR, immunofluorescence, and immunoblotting. Normally, MEF2 activity (Fig. S1A) (5, 6) and protein of the MEF2 isoforms (2, 12) are widely distributed in specific patterns in the brain. In adult n-Cre+/Mef2cloxp/Δ2-null mutant mice, we observed a marked decrease in brain size, cortical thickness (Fig. S1B), and body weight (Fig. S1C). Nonetheless, during brain development, we found no change in cell proliferation (Fig. S1D) or apoptosis (by TUNEL) in n-Cre+/Mef2cloxp/Δ2-null mice versus control. Notably, only 60% of the conditional null mice survived to adulthood (Fig. S1E).
Newly Formed Cortical Neurons Are Abnormally Compacted in MEF2C Conditional Knockout Mice.
In mouse neocortex, neuronal differentiation commences at approximately E11.5, peaks at approximately E15.5, and reaches completion approximately at birth (13). Excitatory pyramidal neurons make up ≈70–80% of the neuronal population and migrate radially during corticogenesis. The other 20–30% of neocortical neurons are GABAergic interneurons generated in the ganglionic eminence; they enter the neocortex through tangential migration. Once within the neocortex, interneurons undertake radial migration together with pyramidal neurons to form the characteristic laminated cortical plate. In the n-Cre+/Mef2cloxp/Δ2-null neocortex, we found that during this migration of neurons, severe compaction occurred in the cortical plate with variable phenotypic penetrance. In the severe manifestation, we observed dramatically compacted clusters of neurons, resulting in disrupted layer formation at a late embryonic stage (Figs. 1 and 2 and Fig. S2) (n = 10 mice at E18.5).
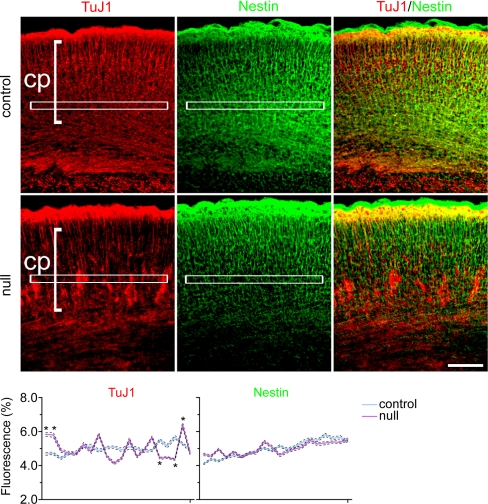
Fig. 1.
At E18.5, in the absence of MEF2C, migrating neurons formed abnormal clusters above the subplate. Staining for committed neuronal markers Nestin and TuJ1 (TuJ1+) in the cortical plate (cp) is shown. Graph quantifies immunofluorescent markers in control versus Mef2c-nulls (*, P < 0.001; see Materials and Methods).
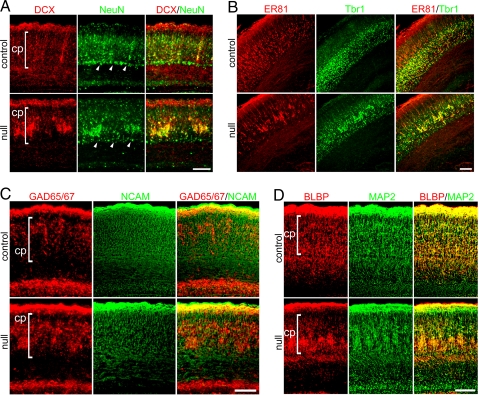
Fig. 2.
Severe E18.5 Mef2c-null phenotype shows altered organization in neocortex. (A) The immature neuronal marker DCX and mature neuronal marker NeuN were expressed by aberrantly clustered neurons in Mef2c-null neocortex. Arrowheads indicate the subplate. (B) Layer 5-specific marker Er81 and layer 6-specific marker Tbr1 were expressed by aberrantly clustered neurons in Mef2c-null neocortex. (C) GAD 65/67-positive mature interneurons clustered within the cortical plate; PSA-NCAM-positive proliferating neuronal progenitor cells showed a normal distribution in the Mef2c-null. (D) The morphology of radial glia labeled by BLBP was extremely aberrant in the severely affected phenotype of the Mef2c-null neocortex with a pattern similar to that of MAP-2-stained migrating neurons.
We also investigated the number and distribution of newly generated neurons. By stereological counting with the optical dissector, 4 days after bromodeoxyuridine (BrdU) pulse labeling at E14.5, the ratio of newborn neuronal nuclear antigen (NeuN)-expressing neurons to total BrdU-labeled cells in the Mef2c conditional null neocortex was similar to that of control (<3.9% difference), although the neurons were distributed in a more compacted pattern (Fig. 2A and Fig. S2). Despite this compaction, compared with control, the Mef2c conditional null neocortex manifested a normal laminar pattern of cells born between E12.5 and E15.5 (Fig. S3).
Loss of MEF2C Affects Distribution of Postmitotic Neurons Rather than Neural Precursor Cells During Embryonic Development.
In conditional null mice at E18.5, nestin- and PSA-NCAM-expressing neural stem/progenitor cells appeared to be unaffected (Figs. 1 and 2C), suggesting that despite compaction of a population of migrating cells, the precursor cells were distributed normally throughout the neocortex. In contrast, compacted cells had further committed to a neuronal fate, expressing the early neuronal markers β-III tubulin (TuJ1) and doublecortin (DCX) (Figs. 1 and 2A); subsequently, they expressed the more mature neuronal markers NeuN, ETS class transcription factor (Er) 81, T-box brain gene (Tbr) 1, glutamate decarboxylase (GAD) 65/67, and microtubule-associated protein 2 (MAP-2) (Fig. 2). Subplate formation appeared to be intact, consistent with normal neural stem/progenitor cell (NSC) development between E11 and E13. In the more severely affected cases, radially migrating neurons in the E18.5 Mef2c-null neocortex appeared to be hindered by compaction soon after crossing the subplate (Fig. 2A Middle and Fig. S2). Both pyramidal neurons and interneurons were affected, as indicated by the specific markers Tbr1 and GAD 65/67, respectively (Fig. 2 B and C). In less severely affected cases at E18.5, many cortical plate neurons remained ectopically distributed between the subplate and cortical plate (Fig. S4 Left).
We next wanted to rule out the involvement of known signaling pathways that affect migration during neocortical development in the Mef2c brain null phenotype. For example, DCX is involved in cortical neuronal migration (14), but we found normal expression of this early neuronal marker in the compacted cells (Fig. 2A). Also, the Reelin- and CDK5-signaling pathways are well known for regulating radial migration; the order of neocortical layer formation becomes inverted when either pathway is disrupted (15). So, we examined Disabled 1 (Dab1), which mediates Reelin signaling, and p35, which is involved in CDK5 signaling, but we found that both were also expressed in the compacted cells in the Mef2c conditional null cortex (Fig. S5). These Dab1 and p35 findings are consistent with the normal ordering of the layers after migration in our conditional null neocortex (Fig. S3). Thus, the compacted cell phenotype in the Mef2c-null mutant does not appear to be caused by a cell intrinsic migrational defect of these molecules. Additionally, because of the abnormal neuronal clustering in the more severely affected neocortices of the Mef2c-null mutants, we checked two cell adhesion molecules known to be important in this process, but they were expressed in the mutant mice as well (Fig. 2C for PSA-NCAM and Fig. 3A for integrin α5).
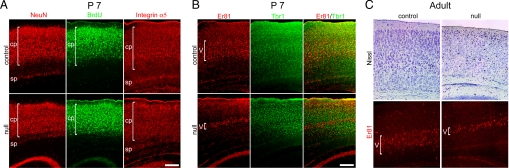
Fig. 3.
Mef2c-null mice at P7 and adult manifest a disorganized, more compacted cortical plate that lies abnormally close to the subplate. (A) NeuN and integrin α5 staining of neurons, labeled with BrdU upon their generation at E14.5, reveals the disorganized cortical plate in the null mice. (B) Layer 5 labeled with Er81 showed aberrant neuronal migration and little colocalization with Tbr1. (C) Similar to P7, in adult mice Nissl and Er81 staining reveal that Mef2c-null neocortex manifested a disorganized and compacted cortical plate, with layer 5 most affected. cp, cortical plate; sp, subplate. (Scale bars, 200 μm.)
Classically, radial glia were thought to serve as a scaffold for migrating neurons in radial migration. More recently, radial glia were shown to divide asymmetrically to replenish themselves and at the same time produce neural progenitor cells that stain positively for brain lipid-binding protein (BLBP), glial acid fibrillary protein (GFAP), vimentin, and nestin (16, 17). At E18.5, we found cells staining for these markers that followed the severity of the phenotype (e.g., Fig. 2D shows clustering of BLBP in mice with the severe phenotype; Fig. S4 shows less clustering but clearly an altered expression pattern of vimentin in mice with the less severe phenotype). Thus, the morphology of BLBP- and vimentin-labeled radial glia reflected the abnormal compaction and neuronal distribution in the Mef2c-null neocortex, suggesting that radial glia could contribute to these defects.
Mef2c-Null Mice Manifest Disorganization of the Cortical Plate in Postnatal/Adult Neocortex.
In mice, neuronal migration is largely complete, and all cortical layers and areas have formed by postnatal day (P)7, although the brain is not considered to be fully mature until 3 weeks of age (18). Here, we found that the developmental abnormality of neuronal compaction that we observed in embryonic Mef2c-null mice (Fig. S6) led to disorganization of the cortical plate by P7 and persisted into adulthood, as shown by neuron-specific staining for NeuN, integrin α5, Er81, and Tbr1, and by Nissl staining and BrdU incorporation (Fig. 3). In these mice, the cortical plate did not separate well from the subplate, and neuronal cell distribution within the neocortex manifested a more compacted, thinner cortical plate (Fig. 3). This compaction of neurons in the laminar cortex persisted from postnatal through adult stages (number of mice examined, n = 25 at P7 and n = 43 for adult). In particular, layer 5 (specifically labeled with Er81) was dramatically thinned and more compacted in the Mef2c-null mutants (Fig. 3 B and C). Furthermore, the neurons were smaller in size as assessed quantitatively on Nissl-stained sections [18.52 ± 2.89 μm in diameter for control (n = 84 cells) versus 15.00 ± 1.90 μm for Mef2c-null (n = 92); mean ± SD, P < 0.0001] (Fig. 3C), suggesting a less mature phenotype in the knockout. By stereological counting, total NeuN-labeled neurons in the entire adult cortex were decreased by ≈30% in the Mef2c-null, compared to WT.
Mef2c-Null Neurons Exhibit Immature Electrophysiological Properties.
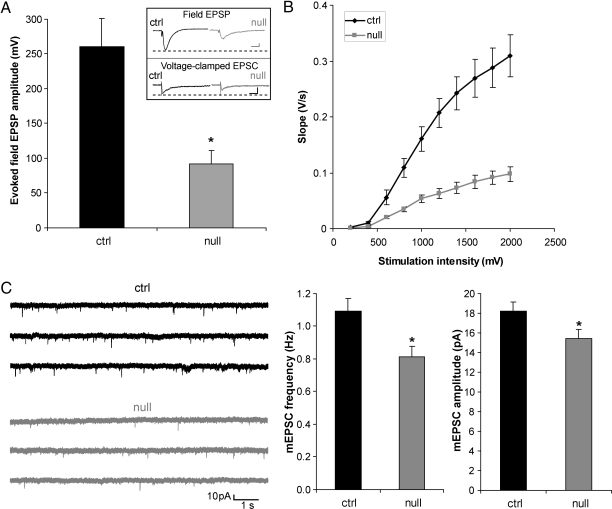
We next asked whether neurons in the adult cortex exhibit more immature electrophysiological properties than their WT counterparts. MEF2-binding sites are present in the promoters of many neuronally restricted genes. These include genes involved in neuronal electrical activity, such as type II sodium channels, AMPA receptor subunits, and NMDA receptor subunits (19, 20); thus, less transcription could conceivably contribute to the observed lower NR1 protein expression (Fig. S7). Therefore, lack of MEF2C early in development might be expected to result in a more immature electrical phenotype in the adult. In fact, we found in multielectrode array (MEA) recordings that adult hippocampal slices from Mef2c-null brains compared with WT showed smaller field excitatory postsynaptic potentials (fEPSPs) and smaller input/output (I/O) ratios, characteristic of an immature neuronal network (Fig. 4 A and B). In a parallel fashion, patch–clamp experiments of single neurons in acute brain slices of Mef2c-null mice showed that layer 5 of the adult cortex displayed a decrease in the frequency and amplitude of miniature (m)EPSCs (Fig. 4C), smaller evoked excitatory postsynaptic currents (EPSCs), and a decrease in the I/O ratio compared with WT (Fig. S8). One possibility to explain the smaller amplitude of EPSC(P)s, decreased frequency, and amplitude of mEPSCs, and smaller I/O curves is that fewer synapses and postsynaptic receptors/channels are found in the adult Mef2c-nulls, for example, as encountered in the early stages of initial synapse formation. To investigate further the potential presynaptic consequences of early knockout of MEF2C, we measured paired pulse facilitation (PPF) in cortical neurons. PPF represents short-term enhancement of presynaptic function in response to the second of two paired stimuli caused by residual Ca2+ in the presynaptic terminal after the first stimulation. For example, increased PPF is associated with a decrease in the probability of neurotransmitter release. We observed no statistical difference in PPF in the Mef2c-null and WT mice but a trend toward increased PPF in the nulls (Fig. S9), suggesting that a decrease in neurotransmitter release might contribute to the decrease in mEPSC frequency in a minor way, whereas the major effect might be accounted for by a decrease in synaptic number. Quantitative synaptophysin staining showed that fewer synaptic sites were indeed present in knockout mice (Fig. S10). At the same time, MAP-2 staining of the neuropil held constant between Mef2c-null and WT mice, suggesting that frank neurodegeneration was not responsible for the decrease in synapse number. These data are consistent with the notion that adult Mef2c-null neuronal networks manifest a more immature phenotype than WT.
Fig. 4.
Reduced excitability of Mef2c-null adult brain slices compared with control. (A) fEPSPs from hippocampal slices stimulated at the Schaeffer collateral afferent pathway and recorded in CA1 stratum radiatum. Responses of WT (ctrl) pyramidal neurons, as measured by initial slope, were significantly larger than those of Mef2c-null at 30% maximal stimulation intensity (n = 11; P < 0.05). (Inset Upper) Representative fEPSPs from hippocampal slice in MEA recordings. (Scale bar, 100 ms and 50 mV.) (Lower) Representative evoked EPSCs from layer 5 neocortex in whole-cell recordings at a holding potential of −70 mV (Scale bar, 50 ms and 100 pA.) (B) I/O curves of hippocampal fEPSP initial slope in response to increasing stimulation intensity. (C) Representative mEPSCs recorded from Mef2c-null and WT layer 5 pyramidal cells voltage clamped at −70 mV. Bar graphs show quantification of results. mEPSC frequency was decreased in Mef2c-null compared with WT cortical neurons (n = 33; *, P < 0.005), consistent with a decrease in synaptic release sites or probability of release.
Mef2c-Null Mice Display Behavioral Phenotypes.
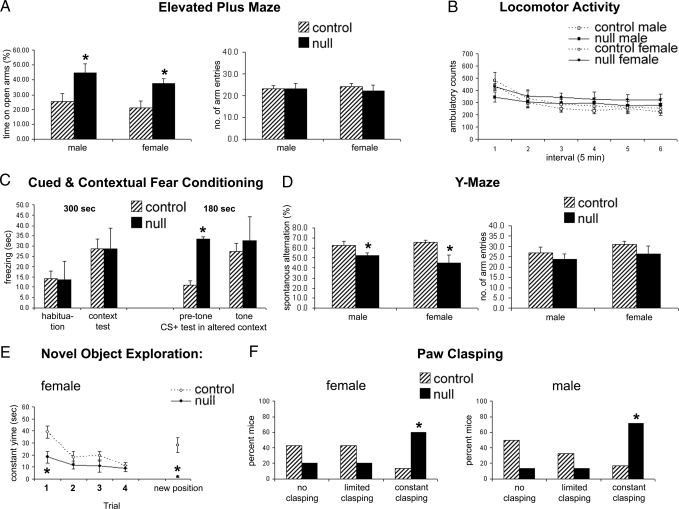
To determine whether the apparent electrical immaturity during electrophysiological recordings was reflected in neurological function of Mef2c brain nulls, we performed a series of behavioral tests on mice that survived to adulthood. The behavioral tests demonstrated that adult Mef2c brain null mutant mice displayed abnormal anxiety-like behaviors, decreased cognitive function, and marked paw wringing/clasping stereotypy. For example, the Elevated Plus Maze Test predicts how animals respond to an approach–avoidance situation involving open and elevated spaces versus enclosed “safe” areas. Mef2c conditional null mice spent significantly more time than littermate controls on the open arms [F(1,36) = 7.5; *, P < 0.01 by ANOVA] while showing no difference in total arm entries (Fig. 5A). These data suggest that the KO mice have altered anxiety-like behavior with no overall impairment of mobility. This conclusion is also supported by the results of the Locomotor Activity Test in which there was no overall effect of genotype or genotype × sex in ambulatory scores across the test session (Fig. 5B). Interestingly, Mef2c conditional null mice had less activity in the center of the apparatus and less rearing behavior than littermate control mice [F(1,34) = 13.9; *, P < 0.001], supporting the presence of an altered anxiety response in these mice (Fig. S11).
Fig. 5.
Mef2c-null mice display altered anxiety-like behavior and decreased cognitive function. (A) The Elevated Plus Maze showed an apparent lowered state of anxiety without loss of function in mice lacking MEF2C compared with controls based on an increase in the percentage of time spent on the open arms but no difference in total arm entries, calculated over a 5-min period. (B) Locomotor activity was not different among null and control groups measured for 30 min as horizontal (locomotion) behavior. (C) Cued and contextual fear conditioning tests revealed that Mef2c-null mice manifest an altered anxiety phenotype, displaying freezing behavior in the altered context before tone onset [t(31) = 2.2, P < 0.05 by ANOVA; n ≥ 10 mice for each paradigm in A–F). Values are mean ± SEM. (D) Total number and order of arm entries on the Y maze were determined in a single 5-min test. (E) Novel object exploration was measured as contact time in one location for four 5-min trials and then a fifth trial with the object moved to a different spatial location. (F) For the paw-clasping observation, mice were suspended by their tails, observed for 10 s, and rated for clasping.
In the Fear Conditioning Test, Mef2c conditional null mice showed increased freezing in a novel environment (altered context) before the conditioned stimulus (represented by the onset of a tone) (Fig. 5C). This effect was not caused by a generalized freezing phenotype in these mice because they displayed normally low levels of freezing during the habituation trial. These data may be explained by the shock (the unconditioned stimulus) resulting in a generalized fear behavior. This confounded the analysis of cued conditioning because levels of freezing before cue exposure were elevated. The results of the context portion of the Fear Conditioning Test were consistent with a deficit in spatial memory (Fig. 5C). Although there was an overall increase in freezing in the context previously associated with shock (P < 0.05), if the genotypes were investigated separately, this effect was significant in WT controls (P = 0.01) but not in the Mef2c-null mice (P = 0.2).
We performed the Y Maze Test for Spontaneous Alternations, which measures the tendency to make free choices in an alternating manner in a Y maze. This is a paradigm for studying working memory. Mef2c conditional null mice showed a decrease in spontaneous alternations [F(1,36) = 11.2; *, P < 0.01], with no significant difference in total arm entries (Fig. 5D). These results suggest that Mef2c conditional null mice have impaired spatial working memory. We also performed the Novel Object Exploration Test to measure the ability of mice to build up spatial representations of their environment and react to the introduction of novel stimuli. Littermate control mice showed the classic pattern of habituation to the object over the four initial trials and then renewed interest in the object when it was moved to a new location. In contrast, Mef2c conditional null mice displayed low levels of object exploration, again consistent with altered anxiety-like behavior. The nulls showed no evidence of renewed interest in the object when it was moved, suggesting decreased spatial memory functioning in these mice (*, P < 0.05) (Fig. 5E). Intriguingly, paw clasping was observed to occur spontaneously in the Mef2c-null mice when lifted by the tail. Therefore, we examined this behavior more quantitatively. Mef2c conditional null mice showed significantly more paw clasping than littermate control mice (χ2 = 7.6; *, P < 0.05 by χ2 test) (Fig. 5F). This paw-clasping behavior may model hand wringing behavior in humans, as seen in Rett syndrome.
Discussion
The processes by which neurons are born, migrate, differentiate, and integrate into neural circuits are central problems in the study of brain development. An understanding of these processes would advance knowledge of general neurodevelopment and provide valuable knowledge concerning how such processes might produce pathology when alterations occur. We report here that the transcription factor MEF2C is crucial for normal neuronal development, distribution, and electrical activity in the neocortex. When MEF2C was absent early in brain development, we observed an abnormal distribution of neurons in the cortical plate with consequent developmental abnormalities. Hippocampal and cortical electrophysiological responses coupled with quantitative immunohistofluorescence suggested the presence of a more immature neuronal network with fewer synapses. Importantly, this result is the exact opposite of that obtained by knocking down MEF2 activity in more mature neurons, which resulted in an increase in synapse activity and number (1).
The spatial cognitive deficits observed in Mef2c-null mice across three behavioral tests are consistent with hippocampal deficits, and the spontaneous paw-clasping behavior suggests motor control deficits. Interestingly the behavioral findings are similar to those observed in two mouse models of a mutant Mecp2 gene, encoding methyl-CpG-binding protein 2 (MeCP2) (21–25). This pattern of altered anxiety, reduced cognition, and hand wringing are typical of Rett syndrome, which is generally caused by genetic mutation of the Mecp2 gene. MeCP2 is involved in gene silencing and known to affect the activity of brain-derived neurotrophic factor (BDNF) (21, 26). In turn, BDNF has recently been shown to activate MEF2 transcription factors (1, 9), raising the possibility of MEF2C involvement downstream in the disease process to effect aspects of the Rett phenotype. Additionally, the Mecp2 promoter contains multiple putative MEF2 sites (22), consistent with the notion that MEF2C may provide feedback to activate Mecp2 transcription. Note, however, that because Mef2c is autosomal, its deletion does not result in an X-linked pattern of inheritance as seen in Rett syndrome.
The putative role of MEF2C in disease notwithstanding, our knockout of Mef2c very early in brain development at the NSC stage produces a “compaction defect” during early neuronal differentiation and results in a “decreased synaptic phenotype”' with behavioral deficits in adulthood suggestive of an autism-like syndrome. We speculate therefore that such early knockout of Mef2c contributes to an adult phenotype suggestive of immaturity or developmental arrest in the neocortex, stunting somal size, number, distribution, and synapse formation. Intriguingly, Eric Olson and colleagues (27) observed that when Mef2c is knocked out in vivo at a later time point in development under the GFAP promoter-driven Cre transgene, an “increased synaptic phenotype” results, similar to reported MEF2 knockdown experiments performed on maturing cortical neurons in vitro (1). Taken together, these two sets of findings can be understood in the context of representing critical pleiotropic effects of MEF2C on disparate temporal points in the neurogenic program.
Materials and Methods
Generation of Mef2c Conditional Null Mice and MEF2 Reporter Mice.
All experiments were performed in accordance with institutional guidelines concerning care and treatment of vertebrate animals. Refer to SI Materials and Methods for additional details of the mouse strain crosses and other methods used in this work.
Quantitative Immunohistochemistry for NSC and Neuronal Markers.
Quantification of immunofluorescent signals was carried out by obtaining images of the developing neocortex by deconvolution microscopy and dividing them into a series of bands extending from the ventricular zone to the cortical surface.
Stereological Neuronal Cell Counts.
Cells were pulse-labeled with BrdU at E14.5 and analyzed through serial sections at E18.5 for the percentage of BrdU-positive cells that were also NeuN-positive. In the adult, total NeuN-positive cells were counted with an optical dissector technique.
Quantitative Immunohistochemical Analysis for Volume of Dendritic Neuropil and Synapse Number.
For assessment of neuronal somal/dendritic and synaptic changes, sections were immunolabeled with antibodies against MAP-2 and synaptophysin, respectively.
Electrophysiological Techniques.
Brains of neonatal and adult male mice were analyzed by using MEA or patch–clamp recordings.
Neurobehavioral Tests.
We measured the effects on the mice of the Mef2c gene knockout by assessing their paw-clasping response while being held by the tail and by using well accepted behavioral tests.
Statistical Analysis.
Data are presented as means ± SEM, unless otherwise indicated. Comparisons were made by using a one-tailed Student's t test or an ANOVA for multiple comparisons. Values for n and significance limits are indicated in figure legends or the text.
Note Added in Proof.
After the acceptance of this manuscript, Chahrour et al. (28) reported that Mef2c is one of a group of genes that is regulated by MeCP2. Taken together with our work, this molecular link is consistent with the notion that MEF2C may play a role in the pathogenesis of Rett syndrome and possibly other forms of autism.
Supplementary Material
Acknowledgments.
We thank members of the Lipton laboratory for helpful suggestions, J. Cui and J.C. Piña-Crespo for help with experiments, and Eric Olson for exchanging information before publication and for sharing his MEF2 reporter mice. S.A.L. was a Senior Scholar in Aging Research of the Ellison Medical Foundation. This work was supported in part by National Institutes of Health (NIH) Grants P01 HD29587 and R01 NS044326 (to S.A.L.) and Fellowship Award 0625088Y from the American Heart Association (to J.C.R.). The support of facilities by the La Jolla Interdisciplinary Neuroscience Center Cores, NIH Blueprint Grant P30 NS057096, is kindly acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802876105/DCSupplemental.
References
- 1.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 2.Leifer D, et al. MEF2C, a MADS/MEF2 family transcription factor expressed in a laminar distribution in cerebral cortex. Proc Natl Acad Sci USA. 1993;90:1546–1550. doi: 10.1073/pnas.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JF, Schwarz JJ, Olson EN. Myocyte enhancer factor (MEF) 2C: A tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci USA. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmondson DG, et al. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 5.Lyons GE, et al. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci. 1995;15:5727–5738. doi: 10.1523/JNEUROSCI.15-08-05727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naya FJ, et al. Transcriptional activity of MEF2 during mouse embryogenesis monitored with a MEF2-dependent transgene. Development. 1999;126:2045–2052. doi: 10.1242/dev.126.10.2045. [DOI] [PubMed] [Google Scholar]
- 7.Lin Q, et al. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto S, et al. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci USA. 2000;97:7561–7566. doi: 10.1073/pnas.130502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalizi A, et al. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, et al. Myocyte enhance factor 2C as a neurogenic and antiapoptotic transcription factor in murine embryonic stem cells. J Neurosci. 2008;28:6557–6568. doi: 10.1523/JNEUROSCI.0134-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Shah S, Bulleit RF. The expression of MEF2 genes is implicated in CNS neuronal differentiation. Brain Res Mol Brain Res. 1996;42:307–316. doi: 10.1016/s0169-328x(96)00135-0. [DOI] [PubMed] [Google Scholar]
- 13.Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12:244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 14.Gleeson JG, et al. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 15.D'Arcangelo G, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 16.Fishell G, Kriegstein AR. Neurons from radial glia: The consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 17.Gotz M, Hartfuss E, Malatesta P. Radial glial cells as neuronal precursors: A new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res Bull. 2002;57:777–788. doi: 10.1016/s0361-9230(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 18.Chenn A, et al. New York: Oxford Univ Press; 1997. Development of the Cerebral Cortex: Mechanisms Controlling Cell Fate, Laminar and Areal Patterning, and Axonal Connectivity. [Google Scholar]
- 19.Chen Y, et al. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 21.Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Chen RZ, et al. Deficiency of methyl-CpG-binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 23.Pelka GJ, et al. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- 24.Shahbazian M, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 25.Stearns NA, et al. Behavioral and anatomical abnormalities in Mecp2 mutant mice: A model for Rett syndrome. Neuroscience. 2007;146:907–921. doi: 10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbosa AC, et al. MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci USA. 2008;105:9131–9396. doi: 10.1073/pnas.0802679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.