Abstract
Species with “slow” life history strategies (long life, low fecundity) are thought to produce high-quality offspring by investing in larger, but fewer, young. Larger eggs are indeed associated with fewer eggs across taxa and can yield higher-quality offspring. Tropical passerines appear to follow theory because they commonly exhibit slow life history strategies and produce larger, but fewer, eggs compared with northern species. Yet, I show here that relative egg mass (corrected for adult mass) varies extensively in the tropics and subtropics for the same clutch size, and this variation is unexplained. I propose a hypothesis to explain egg size variation both within the tropics and between latitudes: Relative egg mass increases in species with cooler egg temperatures and longer embryonic periods to offset associated increases in energetic requirements of embryos. Egg temperatures of birds are determined by parental incubation behavior and are often cooler among tropical passerines because of reduced parental attentiveness of eggs. Here, I show that cooler egg temperatures and longer embryonic periods explained the enigmatic variation in egg mass within and among regions, based on field studies in tropical Venezuela (36 species), subtropical Argentina (16 species), and north temperate Arizona (20 species). Alternative explanations are not supported. Thus, large egg sizes may reflect compensation for increased energetic requirements of cool egg temperatures and long embryonic periods that result from reduced parental attentiveness in tropical birds.
Keywords: clutch size, incubation, life history, passerines
Understanding why species differ in the number and size of offspring is a central question of life history theory because these traits exert a major influence on fitness (1, 2). Indeed, egg and yolk mass can strongly affect offspring fitness across taxa (2, 3). Larger eggs and higher-quality offspring are thought to be favored for competitive environments and associated “slow” life history strategies (i.e., long life, slow development, low fecundity) (1–3) that often typify tropical species (4). These larger young are expected to come at the expense of fewer young, and this tradeoff has been widely observed among taxa other than birds (1–3). In birds, a tradeoff between egg size and number is often weak or nonexistent (2), such that egg size variation is puzzling. Tropical and subtropical passerines, however, appear to follow theory because they commonly exhibit slow life histories and produce larger eggs associated with smaller clutch sizes compared with north temperate species (4). Yet, this apparent affirmation of theory may be an illusion because relative egg mass (corrected for body mass) varies extensively in the tropics whereas clutch size does not (Fig. 1), and this egg size variation is unexplained. I propose a hypothesis for egg size variation within and among latitudes for birds and other organisms with ectothermic embryos: egg mass is relatively larger in species with greater energetic requirements of embryos experiencing cooler egg temperatures and longer embryonic periods.
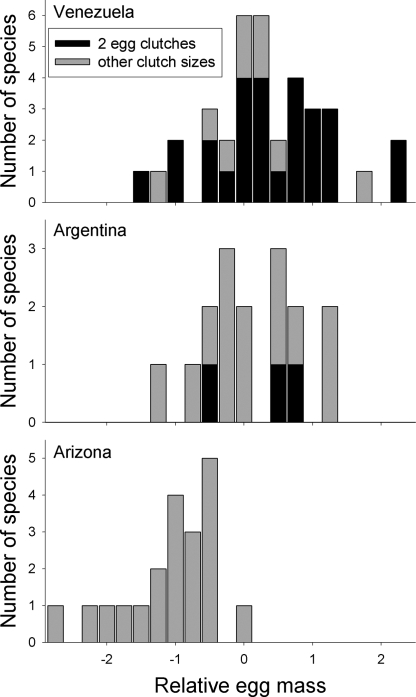
Fig. 1.
Distribution of variation in relative egg mass (corrected for adult mass) among 72 passerine species studied at three sites: tropical Venezuela, subtropical Argentina, and north temperate Arizona. Relative egg masses for species with a clutch size of two eggs (i.e., mean clutch size >1.9 and <2.1 eggs) are indicated by black bars to illustrate that the full range of variation in relative egg mass exists for a constant clutch size.
This hypothesis has a clear mechanistic basis. Embryonic growth efficiency, defined as the proportion of energy converted to tissue, is affected by egg temperature in ectotherm species (5, 6). Growth efficiency is generally maximized at some temperature that is species-specific, and more energy is required by the embryo from reduced growth efficiency at warmer or cooler temperatures (5, 6). Embryos of many ectotherm species experience lower growth efficiency and require more energy in colder environments that thereby yield longer embryonic periods and smaller and lower-quality hatchlings (5–10). Bird embryos are ectothermic, especially during early incubation, and embryos develop best at quite high (i.e., 35.5–39.5°C) temperatures associated with the high body temperatures of birds (11–15). Even maximum ambient temperatures in midelevation tropics (e.g., 25°C) are quite cold relative to bird embryonic development (16). As a result, periodic absences (i.e., inattentiveness of eggs) by incubating parents during the daytime (parents incubate all night) cause relatively cold egg temperatures (14–16). Absences are commonly longer among tropical birds, causing egg temperatures to commonly drop as low as 18°C and average colder temperatures than north temperate relatives (16). Colder egg temperatures cause greater energy requirements by embryos and reduced growth efficiency that yield smaller offspring (11–13) and longer embryonic periods (16). Moreover, longer embryonic periods also increase the amount of energy needed by the embryo (13, 17). Larger eggs, therefore, may be needed to provide resources to offset increased energy requirements of embryos from parental inattentiveness and longer embryonic periods that are especially common in the tropics (see ref. 16).
Extra resources are reflected only by relative egg size; egg size increases with adult mass (2, 4) such that correcting for adult mass is necessary to show that eggs are larger than expected for the size of the species. Thus, under the hypothesis proposed here, relative egg size should be larger in species with cooler average egg temperatures.
Relative egg size also should be larger in species that have relatively long embryonic periods from cooler egg temperatures, under the hypothesis here. Like egg size, embryonic period needs to be corrected for any allometry effects (18–21), because effects of colder temperature should be expressed as embryonic periods that are longer than expected for their size (16). However, passerines provide a unique opportunity to test this prediction. Embryonic period does not increase with body mass within passerines because of overriding effects of parental behavior and egg temperature (16, 22). The great variation in egg temperatures and associated embryonic periods among passerines that is independent of size (16) allows a clear and strong test of the influence on relative egg mass variation. Thus, I tested the predictions of my hypothesis with data on relative egg size (corrected for adult mass), clutch size, egg temperature, and embryonic period (4, 16) from subsets of 72 species of passerines studied in tropical Venezuela (36 species), subtropical Argentina (16 species), and north temperate Arizona (20 species).
Results
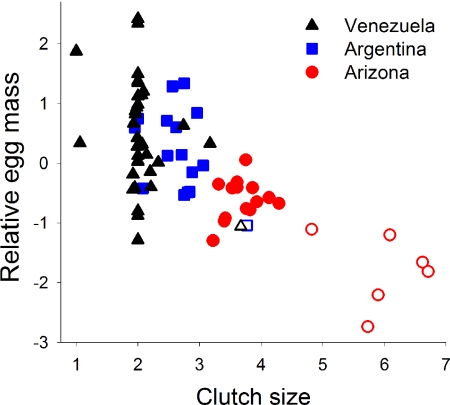
Relative egg mass differed among sites (site: F2,69 = 20.6, P < 0.001), with smaller eggs (Fig. 1) in north temperate Arizona than in tropical Venezuela or subtropical Argentina [least square difference (LSD), P < 0.001 in both cases], but Venezuela did not differ from Argentina (LSD, P = 0.31). Tropical passerines included 72% of the total range of variation in relative egg mass among the three sites (Fig. 1), despite little variation in clutch size (Fig. 2). In fact, the coefficient of variation in relative egg mass for tropical species with a clutch size of two eggs (172%, n = 27 species) was more than double the coefficient of variation (73%, n = 20 species) for north temperate species (Fig. 1) that varied substantially in clutch size (Fig. 2). Only 9% of the variation in relative egg mass was explained by clutch size (Fig. 2) within the tropical and subtropical sites (clutch size: r2 = −0.094, F1,49 = 16.9, P < 0.001; site: F1,49 = 0.3, P = 0.6), whereas 48% of the variation in relative egg mass was explained by clutch size when Arizona was included (clutch size: r2 = −0.476, F1,68 = 16.9, P < 0.001; site: F2,68 = 0.5, P = 0.6). Thus, the majority of variation in relative egg mass was unexplained by clutch size, especially in the tropics and subtropics.
Fig. 2.
Relative egg mass (corrected for adult mass) and clutch size of passerine species among three sites. Relative egg mass was greater in species with smaller clutch sizes based on study of 72 passerine species in tropical Venezuela, subtropical Argentina, and north temperate Arizona, but relative egg size varies substantially for a given clutch size in tropical Venezuela and subtropical Argentina. Open symbols are cavity-nesting species.
Bird species that incubated eggs at cooler average temperatures produced relatively larger eggs as predicted by the hypothesis (Fig. 3). This relationship was true even when clutch size was taken into account and both were controlled for each other (egg temperature: F1,32 = 24.0, P < 0.001; clutch size: F1,32 = 7.8, P = 0.009; site: F2,32 = 0.03, P = 0.97). Indeed, the extensive variation in relative egg mass for a constant (i.e., two eggs) clutch size in the tropics (Fig. 2) was strongly related to variation in egg temperature (Fig. 3).
Fig. 3.
Relative egg mass (corrected for adult mass) and average egg temperature over 24-h periods. Relative egg mass is greater in species where parental inattentiveness causes lower egg temperatures based on study of 37 passerine species in tropical Venezuela, subtropical Argentina, and north temperate Arizona. Open symbols are cavity-nesting species and show an interacting effect where their larger clutches are associated with relatively smaller eggs (Fig. 2).
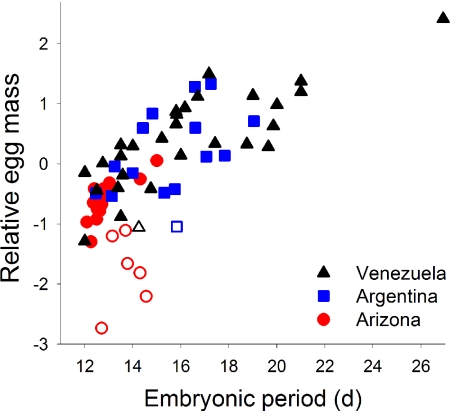
Opposite to allometric expectations, embryonic period was negatively related to log-transformed absolute (not corrected for body mass) egg mass and differed among sites [egg mass: F1,60 = 5.6, P = 0.021; site: F2,60 = 12.8, P < 0.001; interaction not significant; pooled slope = −1.55 ± 0.65 (SE)]. Thus, allometry does not explain embryonic period in these species because variation in egg temperature is of overriding importance in determining embryonic periods (16). In contrast, relative egg mass (corrected for body mass) was strongly explained by embryonic period (Fig. 4) even when accounting for clutch size, as predicted by the hypothesis (embryonic period: F1,59 = 55.0, P < 0.001; clutch size: F1,59 = 33.7, P < 0.001; site: F2,59 = 1.2, P = 0.31).
Fig. 4.
Relative egg mass (corrected for adult mass) is greater in species with longer embryonic periods (d) among 64 passerine species in tropical Venezuela, subtropical Argentina, and north temperate Arizona. Open symbols reflect cavity-nesting species and show an interacting effect where their larger clutches are associated with relatively smaller eggs (Fig. 2).
Discussion
The increase in relative egg mass with decreasing egg temperature (Fig. 3) and increasing embryonic period (Fig. 4) strongly support the hypothesis advanced here. Moreover, the hypothesis and results help to explain why egg mass is often weakly related to clutch size within birds (2) and is generally larger in tropical birds (4). Egg temperature can vary extensively and independent of clutch size (clutch size: F1,33 = 0.0, P = 0.99; site: F2,33 = 2.5, P = 0.09), and thereby can influence variation in egg size independent of clutch size (Fig. 2). At the same time, egg temperatures are colder for many tropical species because of commonly greater parental inattentiveness (16, 22) and, thus, can cause the commonly larger eggs of tropical birds (Fig. 3).
Greater parental inattentiveness during incubation may provide a benefit to adults of long-lived species by enhancing longevity and future breeding opportunities, which may be more important in many tropical than temperate species (22). Yet, the resulting cooler egg temperatures and longer embryonic periods (16) can reduce embryonic growth efficiency and require additional energy resources by bird embryos (11–13). Provisioning of more resources through larger eggs may be a relatively cheap energetic investment by adults, compared with the energetic costs of incubation, that can compensate for increased energetic costs to embryos. An increase in egg size in colder environments also has been observed in true ectotherms, such as amphibians, but is often attributed to an associated increase in adult size (10). Yet, relative egg size (corrected for adult size) also increased in colder conditions where it has been examined (10), which follows from the hypothesis proposed here. Thus, an increase in relative egg size in situations that increase energetic costs to embryos may generally explain egg size variation among birds and other organisms with ectothermic embryos.
The possibility that larger eggs reflect adaptations to reduce cooling is unlikely because total clutch mass, and its effect on thermal inertia, is still substantially less in tropical and subtropical species than in north temperate relatives (4). Moreover, egg mass increases are small relative to cooling rates and the longer absences of tropical parents. Many tropical and subtropical species are off the nest for such long periods that even the very large eggs of the largest tropical species consistently experience colder temperatures than the tiny eggs of the smallest north temperate species that are absent for shorter periods (16). Thus, the association of relatively larger eggs with cooler egg temperatures and longer embryonic periods is better explained by the hypothesis advanced here.
The absence of a relationship between embryonic period and absolute egg mass (this study) or body mass (16, 22) suggests that allometry is not the basis for the relationship between embryonic period and relative egg mass in passerines (i.e., Fig. 4). Indeed, allometry effects are removed in relative egg size; small species can have large relative egg sizes and large species can have small relative egg sizes. Moreover, egg temperature explains most (78%) of the variation in embryonic period in this group (unpublished data and also ref. 16), and experimental egg transfers verified that egg temperature is a causal explanation for some of the variation in embryonic period (16). Thus, embryonic period reflects parental inattentiveness and the relationship with relative egg size is well explained by the hypothesis advanced here. This hypothesis may also explain the broad relationship between relative (corrected for mass) embryonic period and relative egg mass observed across all orders and families of birds (21). Primary allometry effects are removed by correcting for size; allometry should be most clearly expressed when based on absolute size (18–20). Embryonic periods that are longer than expected based on size (i.e., relatively long development) may reflect costs from cool incubation and yield a relationship with relative egg size under the hypothesis here: relatively large eggs should be produced in species with relatively long embryonic periods if long development is caused by cool temperatures.
Various evidence supports this more general application of the hypothesis. At the smallest extreme, Coliiformes have the smallest relative egg sizes and shortest relative embryonic periods among bird orders (21). Under the hypothesis here, these species should exhibit high parental attentiveness (percentage of time parents spend incubating) and high egg temperatures. Indeed, Colius colius in South Africa (see ref. 16 for description of study system) had very high nest attentiveness [98.0% ± (SE) 0.73%, n = 25 nests] because parents share incubation. Moreover, average egg temperature of 36.1 ± (SE) 0.28°C (n = 5 nests, 16 days of sampling) was at a level that predicts their relatively small eggs (see Fig. 3 for comparisons).
At the other extreme, relatively large eggs should reflect a response to cool temperatures that cause long development under the hypothesis here. Megapodiformes are at the extreme end of relatively large eggs and long embryonic periods (21). This group incubates eggs in mounds of vegetation that vary from 31°C to 34°C and likely reflect egg temperature (23). These temperatures are quite cool compared with optimum temperatures for development (12) and study of one megapode, Leipoa ocellata, documented energy costs to embryos from cool egg temperatures (24). Procellariformes are another order with relatively large eggs and long embryonic periods (21), and they fit the hypothesis of showing low incubation temperature from periodic inattentiveness of eggs among most species (25). Thus, data presented here provide strong support for the hypothesis that explains previously enigmatic variation in relative egg mass within passerines, and anecdotal evidence suggests that it might explain broader variation.
Theory predicts that stable and predictable environments, such as the tropics, should favor investment in larger and fewer young to enhance quality of offspring and their ability to compete in such environments (1). Moreover, slower development (i.e., longer embryonic periods) for a constant temperature can allow increased offspring quality and enhanced adult longevity that may be favored in species with slow life history strategies (2, 16, 26). Indeed, birds show species-specific differences in embryonic periods beyond the variation explained by temperature (16). This variation in embryonic periods and egg size that is independent of temperature can reflect species-specific variation in investment in offspring quality (2, 3, 16, 26). On the other hand, increases in length of embryonic periods from cooler egg temperatures arise from slowed or arrested development that reduce growth efficiency through increased loss of energy to maintenance causing embryos to use some portion of the extra resources from larger eggs (11–13). Whether the increase in egg size exceeds the increased energetic costs of cooler egg temperatures and longer embryonic periods by tropical birds cannot be assessed at present.
Studies like those of Olson et al. (13) are needed that expand to compare growth efficiencies and energetic requirements across species with differing embryo temperatures to estimate the extent to which larger eggs compensate or exceed increased energy needs of embryos experiencing cooler temperatures. The influence of egg size versus egg temperature on offspring quality also could be assessed by examining the strength of their associations with measures of offspring quality (e.g., relative size, brain size, or immune function of hatchlings) across species. Metrics of offspring quality measured in nestlings should be positively related to relative egg size if egg size simply reflects investment in offspring quality. However, offspring quality should be unrelated to egg size if egg size simply compensates for temperature. If egg size does not fully compensate for cooler temperature, then an inverse relationship between egg size and offspring quality is expected. If egg size exceeds temperature costs, reflecting both compensation for temperature and increased investment in offspring quality, then metrics of offspring quality should be inversely related to egg temperature when controlled for egg size, and positively related to relative egg size when controlled for egg temperature.
The tradeoff between clutch size and egg mass while controlling for egg temperature may indeed indicate that tropical parents with smaller clutches are increasing investment per offspring to enhance offspring quality, as traditionally thought (1, 2). Yet, relative egg mass varied substantially independent of clutch size, especially in the tropics (Figs. 1 and 2), and this variation was strongly explained by egg temperature and embryonic period (Figs. 3 and 4). More tellingly, many true ectotherms, such as amphibians, show the opposite geographical pattern, where egg size is smaller in the tropics than toward the poles (10). At the same time, embryonic temperature is generally warmer in the tropics because ambient temperature is warmer and has a stronger influence on embryonic temperature because of an absent or reduced role of parental warming (10). Thus, this opposing pattern fits the hypothesis in that eggs are larger where temperatures are colder. Ultimately, larger eggs of passerine birds in the tropics and subtropics may simply reflect advanced compensation for cooler incubation temperatures from greater parental inattentiveness.
Study Areas and Methods
Study sites were high-elevation (2,300 m elevation) mixed forest in Arizona (34° N latitude), Yungas forest (1,000 m) in northwestern Argentina (26° S), and cloud forest (1,350–2,000 m) in the Andes of Venezuela (9° N) (4, 16). Study sites were searched for nests during breeding seasons of 1988–2007 in Arizona, 1997–1999 in Argentina, and 2002–2007 in Venezuela (4, 16). Embryonic development period was quantified as the difference in days between last egg laid and last egg hatched (16). Nests were checked every 2–4 days, but they were checked daily or twice daily near hatching to accurately measure embryonic period (16). Clutch size was measured as the final number of eggs laid and seen on 2 different days (4). Egg mass was taken from nests found during nest building or egg laying. Egg mass was measured within 3 days of clutch completion by using portable ACCULAB electronic balances (0.001-g precision) (4), and data for 18 additional species were included here that were not in a previous analysis (4). Egg temperatures were measured by placing a thermister in the center of one egg and measuring temperature experienced by the embryo every 12 or 24 s in two to nine nests per species over 4 days per nest, on average (16), and data for nine new species were included here that were not included in previous analyses (16).
I calculated relative egg size by regressing log-transformed egg mass and body mass and taking the residuals across all 72 species. Analysis of covariance was used to examine relationships within and among sites. I controlled for possible phylogenetic effects by using independent contrasts (27) based on the CRUNCH option of the program CAIC (28) following ref. 16. Results were the same (i.e., significant relationships of raw data remained significant) when analyzed by using independent contrasts, as typical when a diverse phylogenetic sample is used, so only results from the raw data are presented.
Acknowledgments.
I am grateful to Dan Barton, Ken Dial, John Maron, Winsor Lowe, the editor, and two reviewers for helpful comments on the manuscript and many people who helped in collecting the data reported here. This work was supported by National Science Foundation for the field work in Venezuela and Argentina and by the U. S. Geological Survey Climate Change Research Program and the National Research Initiative of the U. S. Department of Agriculture Cooperative State Research, Education, and Extension Service for the field work in Arizona.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stearns S. Life history tactics: A review of the ideas. Q Rev Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- 2.Roff DA. Life History Evolution. Sunderland, MA: Sinauer; 2002. [Google Scholar]
- 3.Sinervo B. The evolution of maternal investment in lizards: An experimental and comparative analysis of egg size and its effects on offspring performance. Evolution (Lawrence, Kans) 1990;44:279–294. doi: 10.1111/j.1558-5646.1990.tb05198.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin TE, et al. Life history and ecological correlates of geographic variation in egg and clutch mass among passerine species. Evolution (Lawrence, Kans) 2006;60:390–398. [PubMed] [Google Scholar]
- 5.Heming TA. Effects of temperature on utilization of yolk by chinook salmon (Oncorhynchus tshawytscha) eggs and alevins. Can J Fish Aquat Sci. 1982;39:184–190. [Google Scholar]
- 6.Mitchell NJ, Seymour RS. Effects of temperature on energy cost and timing of embryonic and larval development of the terrestrially breeding moss frog. Bryobatrachus nimbus Physiol Biochem Zool. 2000;73:829–840. doi: 10.1086/318097. [DOI] [PubMed] [Google Scholar]
- 7.Hare KM, Longson CG, Pledger S, Daugherty CH. Size, growth, and survival are reduced at cool incubation temperatures in the temperate lizard Oligosoma suteri (Lacertilia: Scincidae) Copeia. 2004;2004:383–390. [Google Scholar]
- 8.Qualls CP, Andrews RM. Cold climates and the evolution of viviparity in reptiles: Cold incubation temperatures produce poor-quality offspring in the lizard, Sceloporus virgatus. Biol J Linn Soc. 1999;67:353–376. [Google Scholar]
- 9.Webb GJW, Cooper-Preston H. Effects of incubation temperature on crocodiles and the evolution of reptilian oviparity. Am Zool. 1989;29:953–971. [Google Scholar]
- 10.Morrison C, Hero J-M. Geographic variation in life-history characteristics of amphibians: A review. J Anim Ecol. 2003;72:270–279. [Google Scholar]
- 11.Hammond CL, Simbi BH, Stickland NC. In ovo temperature manipulation influences embryonic motility and growth of limb tissues in the chick (Gallus gallus) J Exp Biol. 2007;210:2667–2675. doi: 10.1242/jeb.005751. [DOI] [PubMed] [Google Scholar]
- 12.Hepp GR, Kennamer RA, Johnson MH. Maternal effects in wood ducks: Incubation temperature influences incubation period and neonate phenotype. Funct Ecol. 2006;20:307–314. [Google Scholar]
- 13.Olson CR, Vleck CM, Vleck D. Periodic cooling of bird eggs reduces embryonic growth efficiency. Phys Biochem Zool. 2006;79:927–936. doi: 10.1086/506003. [DOI] [PubMed] [Google Scholar]
- 14.Webb DR. Thermal tolerance of avian embryos: A review. Condor. 1987;89:874–898. [Google Scholar]
- 15.White FN, Kinney JL. Avian incubation. Science. 1974;186:107–115. doi: 10.1126/science.186.4159.107. [DOI] [PubMed] [Google Scholar]
- 16.Martin TE, Auer SK, Bassar RD, Niklison AM, Lloyd P. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution (Lawrence, Kans) 2007;61:2558–2569. doi: 10.1111/j.1558-5646.2007.00204.x. [DOI] [PubMed] [Google Scholar]
- 17.Vleck CM, Vleck D, Hoyt DF. Patterns of metabolism and growth in avian embryos. Am Zool. 1980;20:405–416. [Google Scholar]
- 18.West GB, Brown JH, Enquist BJ. A general model for ontogenetic growth. Nature. 2001;413:628–631. doi: 10.1038/35098076. [DOI] [PubMed] [Google Scholar]
- 19.Arendt JD. Adaptive intrinsic growth rates: An integration across taxa. Q Rev Biol. 1997;72:149–177. [Google Scholar]
- 20.Rahn H, Ar A. The avian egg: Incubation time and water loss. Condor. 1974;76:147–152. [Google Scholar]
- 21.Ricklefs RE, Starck JM. Embryonic growth and development. In: Starck JM, Ricklefs RE, editors. Avian Growth and Development. Oxford: Oxford Univ Press; 1998. pp. 31–58. [Google Scholar]
- 22.Martin TE. A new view for avian life history evolution tested on an incubation paradox. Proc R Soc London Ser B. 2002;269:309–316. doi: 10.1098/rspb.2001.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frith HJ. Temperature regulation in the nesting mounds of the mallee-fowl, Leipoa ocellata gould. Commonwealth Sci Ind Res Org Wildl Res. 1956;1:79–95. [Google Scholar]
- 24.Booth DT. Effect of temperature on development of Mallee fowl Leipoa ocellata eggs. Phys Zool. 1987;64:437–445. [Google Scholar]
- 25.Boersma PD. Why some birds take so long to hatch. Am Nat. 1982;120:733–750. [Google Scholar]
- 26.Martin TE, Schwabl H. Variation in maternal effects and embryonic development rates among passerine species. Philos Trans R Soc London Ser B. 2008;363:1663–1674. doi: 10.1098/rstb.2007.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 28.Purvis A, Rambaut A. Comparative analysis by independent contrasts CAIC: An Apple Macintosh application for analyzing comparative data. Comp Appl Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]