Abstract
Learning and memory depend on the activity-dependent structural plasticity of synapses and changes in neuronal gene expression. We show that deletion of the MEF2C transcription factor in the CNS of mice impairs hippocampal-dependent learning and memory. Unexpectedly, these behavioral changes were accompanied by a marked increase in the number of excitatory synapses and potentiation of basal and evoked synaptic transmission. Conversely, neuronal expression of a superactivating form of MEF2C results in a reduction of excitatory postsynaptic sites without affecting learning and memory performance. We conclude that MEF2C limits excessive synapse formation during activity-dependent refinement of synaptic connectivity and thus facilitates hippocampal-dependent learning and memory.
Keywords: synaptic transmission, synaptogenesis, learning deficits
Neurons process and retain information by forming synaptic connections that are modified by the intensity and frequency of their activity. The capacity to regulate the efficacy of synaptic transmission is essential for the continual remodeling of neural networks required for cognitive processes such as learning and memory. Distinct molecular mechanisms control synaptic plasticity associated with the different temporal stages of memory. A short-term process lasting minutes depends on modifications of preexisting proteins, whereas a long-term process lasting hours and days depends on changes in gene expression and protein synthesis (1).
Originally identified as regulators of muscle development, members of the MEF2 (Myocyte Enhancer Factor 2) family of MADS (MCM1, agamous, deficiens, serum response factor) box transcription factors are expressed in overlapping but distinct regions of the CNS that correlate with the withdrawal of neurons from the cell cycle and acquisition of a differentiated phenotype (2). Mef2c is the first of four Mef2 genes to be expressed in the CNS and, in the adult brain, is highly expressed in the frontal cortex, entorhinal cortex, dentate gyrus, and amygdala (3, 4). RNA interference-mediated knockdown of MEF2A and MEF2D in cultured hippocampal neurons increases the number of excitatory synapses and the frequency of miniature excitatory postsynaptic currents (mEPSCs) (5). These alterations depend on the ability of the MEF2 proteins to stimulate neural activity-dependent transcription of target genes (5). In contrast, loss of MEF2A in cerebellar granule neurons results in a decrease in the number of dendritic claws (6).
Here, we present an analysis of the neuronal functions of the Mef2 gene in vivo. Through conditional deletion of Mef2c and expression of a superactive form of MEF2C in neurons of mice, we show that this MEF2 isoform plays an essential role in hippocampal-dependent learning and memory by suppressing the number of excitatory synapses and thus regulating basal and evoked synaptic transmission.
Results
Brain-Specific Deletion of MEF2C.
We deleted Mef2c specifically in the CNS by breeding Mef2cloxP/loxP females (7) to Mef2cKO/+ heterozygous male (8) mice harboring a transgene that expresses Cre recombinase under the control of the human GFAP promoter (hGFAP::Cre), which is expressed in radial glial cells during late embryogenesis (9–11). Interestingly, radial glial cells not only serve as progenitors for many neurons and glial cells but also give rise to adult SVZ (subventricular zone) stem cells that continue to produce neurons throughout adult life (12).
Mice with homozygous deletion of Mef2c in the brain, hereafter referred to as Mef2cBKOKO mice, were viable to adulthood, although they were slightly smaller than WT littermates [supporting information (SI) Fig. S1A]. Mef2cBKO/KO mice displayed impaired balance beam walking by 4 weeks of age (data not shown) and an abnormal hind/forelimb clasping reflex when suspended by the tail at 2–3 weeks of age (Fig. S1B).
Histological analysis by Nissl staining of brain sections revealed no obvious abnormalities in brain architecture in the Mef2cBKO/KO mice (Fig. S2A). Deletion of the Mef2cloxP allele in the brains of mutant mice was confirmed by in situ hybridization and immunostaining (Fig. S2). In WT mice, Mef2c is expressed throughout the cortex, including the frontal and entorhinal cortex, dentate gyrus, and basolateral amygdala. In Mef2cBKO/KO mice, Mef2c expression was almost abolished in the dentate gyrus, amygdala, particularly the dorsolateral amygdaloid nuclei, and the entorhinal cortex, with only a few neurons still expressing MEF2C (Fig. S2E). Residual MEF2C-expressing neurons likely originate from a subpopulation of neuronal precursors that does not express GFAP-Cre. Quantification of Mef2c expression by real-time PCR also showed that Mef2c mRNA was almost undetectable in the Mef2CBKO/KO dentate gyrus and was reduced by 60% in the hippocampus and frontal cortex (Fig. S2D). Overall, the number and the differentiated status of neurons in the mutant hippocampus appeared unaltered as judged by NeuN immunostaining (data not shown).
Mef2cBKO/KO Mice Show Impairment in Hippocampal-Dependent Learning.
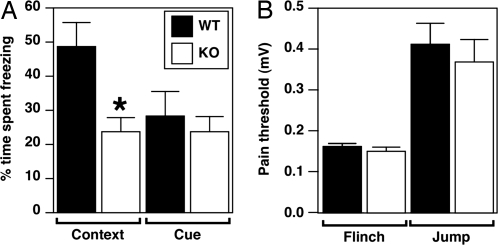
To determine whether Mef2cBKO/KO mice had any alterations in global behavior, we examined the mice in several behavioral paradigms and found no abnormalities in overall locomotor activity or anxiety-related behaviors (Fig. S3). To assess possible defects in learning and memory, Mef2cBKO/KO mice were subjected to a fear conditioning paradigm that assesses contextual- and cue-dependent learning, which are thought to require partially distinct, but overlapping, neural systems (13, 14). Context-dependent fear conditioning requires an intact hippocampus and amygdala, whereas cue-dependent fear conditioning depends only on the amygdala (14, 15). In this paradigm, mice learn to fear an environmental context or an emotionally neutral conditioned stimulus (white noise tone) resulting from its temporal association with an aversive unconditioned stimulus (foot shock). When exposed to the same environmental context (contextual conditioning) or conditioned stimulus (cued conditioning) at a later time, conditioned animals demonstrate a stereotypical fear response, “freezing” (14). Memory for the conditioned stimulus was measured as freezing when presented with the conditioned context or cue in a 24-h retention test. Mef2cBKO/KO mice and WT littermates showed the same level of baseline freezing (data not shown). However, when reexposed to the conditioned context 24 h later, Mef2cBKO/KO mice showed a significant decrease in freezing compared to their WT littermates (Fig. 1A). In contrast, Mef2cBKO/KO mice showed no difference in time spent freezing compared to WT littermates in cue-dependent fear conditioning (Fig. 1A). The threshold responses of the Mef2cBKO/KO mice and WT littermates to foot shock were indistinguishable (Fig. 1B), suggesting that the impairment in context conditioning represents a learning deficit and not a difference in pain sensitivity.
Fig. 1.
Hippocampal-dependent learning deficits in Mef2cBKO/KO mice. Contextual and cued conditioning are two forms of associative learning that induce effective memory for either the context or the cue after a single training session. Memory for the conditioned stimulus was measured as freezing, an absence of visible movement, when presented with the conditioned context or cue in a 24-h retention test. (A) Mef2cBKO/KO (n = 18) showed impaired context-dependent memory compared to WT littermates (n = 17). In contrast, there was no difference in cue-dependent fear conditioning in Mef2cBKO/KO compared to WT mice. (B) The threshold responses of Mef2cBKO/KO and WT mice to foot shocks were indistinguishable, which indicates that the impairment in context conditioning represents a learning deficit and not a difference in pain sensitivity.
In vitro studies have shown that MEF2 proteins are required for the survival of neurons as they differentiate, and that inhibition of MEF2 function by overexpression of a dominant-negative form of MEF2 enhances apoptosis (16, 17). To investigate whether the deficit in hippocampus-dependent learning and memory observed in Mef2cBKO/KO mice is related to an increase in apoptosis, we performed apoptosis assay on sections from 2- and 6-week-old mice using the TUNEL technique. No difference in the number of apoptotic cells was observed between WT and Mef2cBKO/KO hippocampal neurons at postnatal day 14 (P14) or P40 (data not shown). These results suggest that dysregulated cell death in hippocampal neurons is not responsible for the deficit in learning and memory observed in Mef2cBKO/KO mice. These data also indicate that late embryonic deletion of MEF2C does not affect maintenance of hippocampal neurons.
Loss of MEF2C Potentiates the Perforant Path–Dentate Gyrus Synaptic Transmission.
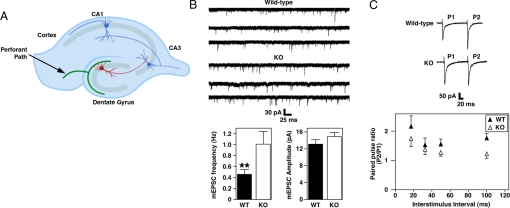
To determine whether the deficit in contextual conditioning is associated with alterations in synaptic function, we quantified the frequency and amplitude of spontaneous miniature excitatory postsynaptic currents (mEPSCs) in the dentate gyrus granule neurons of Mef2cBKO/KO mice and WT controls (Fig. 2). Although the perforant path synapses typically have AMPA and NMDA receptors, mEPSCs recorded in dentate granule cells have a single component mediated by AMPA receptors because the NMDA component is not detectable at rest (18). Interestingly, loss of MEF2C in dentate gyrus neurons caused a doubling of the frequency of mEPSCs (1.01 ± 0.18 Hz) compared to WT neurons (0.45 ± 0.06 Hz) (Fig. 2B). The amplitudes of individual synaptic events in mEPSCs were not affected by the loss of MEF2C, indicating no change in the AMPA receptor-mediated postsynaptic response to spontaneous glutamate release or in the amount of neurotransmitter in each vesicle (Fig. 2B).
Fig. 2.
Basal synaptic transmission and short-term plasticity in dentate granule neurons of Mef2cBKO/KO. (A) Schematic representation of electrophysiological recordings performed on granule cells of dentate gyrus region of mice hippocampal slices. (B) (Upper) Representative traces of whole-cell patch–clamp recordings demonstrating spontaneous EPSCs from dentate granule cells (n = 23 for WT and n = 20 for Mef2cBKO/KO). Increased mEPSC frequency for dentate gyrus neurons in Mef2CBKO/KO mice. **, Statistical significance in pairwise comparison; P = 0.005649. Spontaneuous EPSC amplitudes are unaffected in dentate gyrus neurons of Mef2CBKO/KO mice. (C) Paired-pulse ratios of the first two responses during various stimulation frequencies were measured in WT (filled triangle) and Mef2cBKO/KO (open triangle) dentate gyrus neurons. Paired-pulse facilitation is decreased in Mef2cBKO/KO dentate gyrus when compared to WT mice.
The increase in mEPSC frequency could be due to an increase in the number or density of synapses (19) or to an increase in probability of release at the presynaptic site (20). We investigated whether MEF2C was required for presynaptic function by measuring paired-pulse facilitation (PPF), a short-term enhancement of synaptic activity in response to the second of two paired stimuli due to residual Ca+2 in the presynaptic terminal after the initial stimulus. This form of presynaptic plasticity is associated with the probability of neurotransmitter release (21). PPF was reduced at four time intervals in Mef2cBKO/KO mice compared to WT littermates (Fig. 2C). This decrease in PPF is indicative of an increase in neurotransmitter release and could be partially responsible for the increase in mEPSC frequency in Mef2cBKO/KO dentate gyrus neurons. Collectively, these data indicate that loss of MEF2C in neurons causes alterations in presynaptic function.
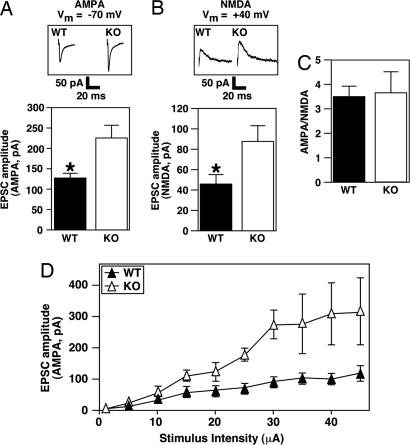
When we stimulated the perforant path and recorded individual neurons in the dentate gyrus of Mef2cBKO/KO and WT littermates, we found an increase in the amplitude of both AMPA- and NMDA-mediated EPSCs in Mef2cBKO/KO neurons (Fig. 3 A and B). The AMPA/NMDA ratios were also similar in WT and Mef2cBKO/KO dentate granule neurons (Fig. 3C). The input–output curve shows that the saturation of AMPA receptor is higher (EPSCs amplitude, 300 pA) in dentate gyrus neurons lacking MEF2C than in WT neurons (EPSCs amplitude, 130 pA) (Fig. 3D). The same was observed for the input–output curve of NMDA receptors (data not shown). These results indicate that ablation of MEF2C during embryonic development potentiates synaptic transmission in the dentate gyrus.
Fig. 3.
Evoked perforant path synaptic transmission in Mef2CBKO/KO. Lateral perforant path was stimulated by a 10- to 40-μA current pulse to evoke ESPCs at perforant path-dentate granule cell synapses. Representative examples of current voltage of glutamate-evoked response from single AMPA (A Upper) or NMDA (B Upper) synapses from WT and MEF2C-deficient neurons. Bar graph showing increased amplitude in both AMPA (A; Vm = −70 mV) and NMDA (B; Vm = +40 mV) mediated evoked EPSCs in granule cells of dentate gyrus of Mef2CBKO/KO mice. Asterisk indicates statistical significance in pairwise comparison: P < 0.0005. (C) The AMPA/NMDA ratios were similar in WT and Mef2CBKO/KO mice. (D) Input–output curves showing increase in the saturation of AMPA receptor in dentate gyrus neurons lacking MEF2C (open triangles).
MEF2C-VP16 Suppresses Excitatory Synapse Number in Vivo Without Affecting Learning and Memory.
To examine whether MEF2 is sufficient to restrain excitatory synapse number in vivo, we expressed a superactive MEF2C-VP16 protein under the control of the rat neuron-specific enolase (NSE) promoter/enhancer, which begins to be expressed shortly after the initiation of synaptogenesis in discrete mature neuronal populations in the cerebral cortex and hippocampus (22). Expression of MEF2C-VP16 was confirmed by in situ hybridization to be confined to subsets of differentiated neurons, mostly in layers III–V of the cerebral cortex and in the CA3, dentate gyrus granular layer (GL), and polymorphic layer (PML) of the hippocampus (Fig. S2Ee). The NSE promoter/enhancer did not drive expression of Mef2c-VP16 mRNA in the entorhinal cortex of the transgenic mice, allowing examination of the consequences of MEF2 activation exclusively in postsynaptic terminals (Fig. S2Ed). The MEF2C-VP16 transgenic mice were indistinguishable from the WT littermates by gross examination, presenting no difference in body weight and a similar level of locomotor activity to the control littermates, and normal motor coordination as assessed by the rotarod test (Fig. S4). In striking contrast to the Mef2cBKO/KO mice, the MEF2C-VP16 transgenic mice did not show any alterations in context-dependent fear conditioning (Fig. S4), suggesting that MEF2C gain of function does not alter hippocampal-dependent learning and memory. These data suggest that MEF2C activity per se is not a direct determinant of learning but presumably facilitates learning-related plasticity by controlling excessive synaptic input.
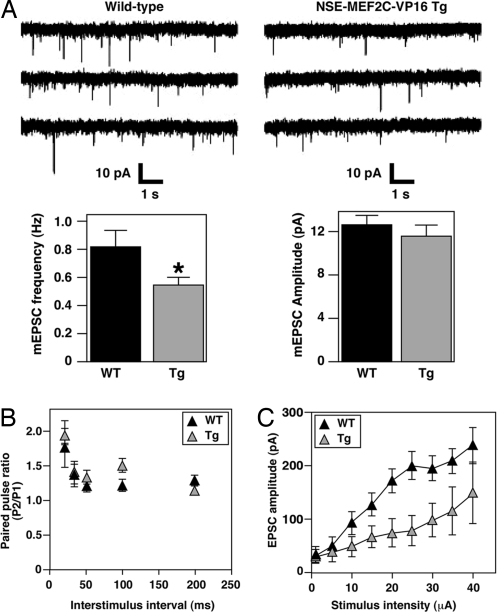
Next, we investigated whether overexpression of MEF2C in dentate granule neurons resulted in functional alterations in synaptic transmission. In contrast to the dentate granule neurons from Mef2cBKO/KO mice, expression of MEF2C-VP16 in dentate granule neurons resulted in a 32% decrease in the frequency of mEPSCs compared to WT littermate controls (0.54 ± 0.06 vs. 0.82 ± 0.11 Hz respectively, P = 0.04) (Fig. 4A). As observed for dentate granule neurons lacking MEF2C expression, the mEPSC amplitude was similar at the transgenic and WT synapses (Fig. 4A). These results imply that MEF2 transcriptional activity is important for basal synaptic transmission in the dentate gyrus. Paired-pulse facilitation measurements appeared normal in the dentate gyrus of NSE-MEF2C-VP16 transgenic mice, indicating normal presynaptic function, and that the decrease in mEPSC frequency is probably due to alterations in synaptic density (Fig. 4B).
Fig. 4.
Synaptic function is altered in NSE-MEF2C-VP16 transgenic mice. (A) (Upper) Representative traces of whole-cell patch–clamp recordings demonstrating spontaneous EPSCs from dentate granule cells (n = 24 for WT and n = 26 for NSE-MEF2C-VP16 transgenic mice). Bar graph showing a decrease in mEPSC frequency for dentate gyrus neurons in NSE-MEF2C-VP16 transgenic mice when compared to WT littermate controls. mEPSC amplitudes are unaffected in dentate gyrus neurons of the transgenic mice. (B) Paired-pulse facilitation at perforant path synapses also appeared normal in the transgenic mice. (C) Input–output curves showing decreased saturation of AMPA receptor in dentate gyrus neurons expressing the superactive MEF2C-VP16.
To corroborate these findings, we recorded AMPA and NMDA receptor-mediated EPSCs upon stimulation of the perforant path. Here, we observed a decrease in the amplitude of both AMPA and NMDA mediated EPSCs in dentate granule neurons expressing MEF2C-VP16 (data not shown), which is contrary to what we found in Mef2cBKO/KO neurons. The saturation of the AMPA receptor was lower (EPSCs amplitude, 130 pA) in dentate gyrus neurons expressing superactive MEF2C-VP16 compared to WT neurons (EPSCs amplitude, 200 pA) (Fig. 4C). Similar results were observed for the input–output curve of NMDA receptors (data not shown). Thus, postsynaptic expression of superactive MEF2C-VP16 resulted in a decreased postsynaptic response to glutamate, decreased mEPSCs frequency, and no change in mEPSCs amplitude. These data are consistent with the interpretation that neurons expressing MEF2C-VP16 have fewer functional postsynaptic sites. In addition, at the time of the expression of MEF2C-VP16, the dentate granule neurons already have established dendrites, extending over the full length of the molecular layer, and axons projecting into the CA3 region. These results indicate that overexpression of MEF2C-VP16 limits synaptic transmission in the dentate gyrus after synaptic connectivity has been established.
Regulation of Dendritic Spine Density in Vivo by MEF2C.
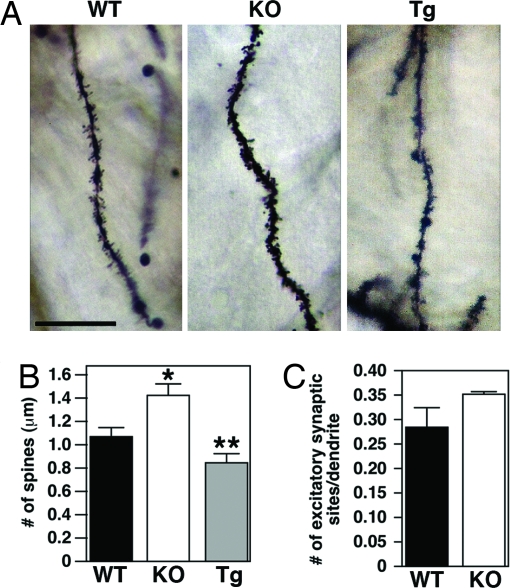
We assessed whether the functional alterations observed in the Mef2cBKO/KO mice were accompanied by physical changes in the dendritic morphology of the dentate granule neurons. Most excitatory synapses in the brain are located on dendritic spines, highly specialized subcellular structures believed to compartmentalize biochemical responses to activation of individual synapses (23). To measure the number of excitatory synapses in dentate gyrus neurons, image stacks from z-series of Golgi-stained tissues were acquired, and the density of dendritic spines was calculated. WT neurons had 1.05 ± 0.34 vs. 1.47 ± 0.28 spines per μm in Mef2cBKO/KO dentate granule neurons, an additional indication of the increased number of excitatory synapses in the Mef2cBKO/KO dentate granule neurons (Fig. 5 A and B). Interestingly, the opposite phenotype was observed in the MEF2C-VP16 transgenic mice, WT dentate gyrus granule neurons contained 1.09 ± 0.33 spines/μm, whereas MEF2C-VP16 transgenic neurons had 0.89 ± 0.20 spines per μm (Fig. 5B). To further address the effect of MEF2C deletion on synapse formation, we examined the number of excitatory synaptic terminals on hippocampal neuron dendrites in culture. WT and Mef2cBKO/KO hippocampal cultures were stained for the excitatory presynaptic marker synapsin-1 and the postsynaptic marker postsynaptic density protein 95 (PSD-95). These proteins were localized to discrete puncta that frequently overlapped, reflecting the clustering of these proteins at synaptic sites. Interestingly, counting the excitatory synapses in P1 hippocampal neurons cultured in vitro for 16 days did not show any significant difference (Fig. 5C). It is noteworthy that at P1, MEF2C expression was already abolished in Mef2cBKO/KO dentate gyrus as detected by immunostaining of coronal hippocampal sections of P1 mice (data not shown). These results indicate that in vivo MEF2 is required to limit excessive increases in the number of excitatory synapses, although it does not hinder synaptogenesis seen during initial development of synaptic connectivity.
Fig. 5.
Effects of MEF2C on excitatory synapse number in dentate granule neurons. Spine density of dentate gyrus dendrites was determined from Golgi-stained sections of (A) Mef2c+/+, Mef2cBKO/KO and MEF2C-VP6 Tg mice. (Scale bar, 10 μm.) (B) Mef2cBKO/KO animals have a significant increase in spine density when compared to WT littermates. *, Statistical significance in pairwise comparison; P = 0.0001. MEF2C-VP16 transgenic mice show a decrease in spine density when compared to WT littermates. **, Statistical significance in pairwise comparison; P = 0.018. For Mef2cBKO/KO mice; P = 0.0001 using two-tailed two-sample equal variance t test; n = 30 dendritic segments from 26 neurons of two mice and n = 27 dendritic segments from 25 neurons of two mice, respectively. For MEF2C-VP16 transgenic mice; P = 0.018 using two-tailed two-sample equal variance t test; n = 28 dendritic segments from 26 neurons of two mice and n = 25 dendritic segments from 23 neurons of two mice, respectively. (C) Average density of synapsin-1-PSD-95 coclusters along the dendrites of cultured hippocampal neurons. There was no significant difference in the number of excitatory synapses between Mef2cBKO/KO and WT neurons.
Normal Synapse Morphology at the Ultrastructural Level in MEF2C Mutant Dentate Gyrus.
Electron micrographs were taken of the dentate gyrus from WT and Mef2cBKO/KO littermates to investigate whether MEF2C-deficient neurons had alterations in synaptic ultrastructure that could account for the increase in excitatory synaptic transmission observed in the Mef2cBKO/KO mice. The number of synaptic vesicles in each synapse, the number of docked vesicles in each active zone, and the length of the postsynaptic densities were not altered in the Mef2cBKO/KO mice (Fig. S5). These results indicate that MEF2C regulates synapse number and synaptic efficacy without affecting synapse structure.
Discussion
In this study, we report on the behavioral and electrophysiological effects of late embryonic deletion of Mef2c in the forebrain and transgenic expression of a superactive form of MEF2C in neurons of mice. In the nervous system, MEF2 transcription factors have emerged as regulators of activity-dependent neuronal survival and differentiation (24). Recently, MEF2A and MEF2D have been reported to negatively regulate excitatory synapse number during synaptic development in vitro (5). In addition, a requirement of MEF2A in the postsynaptic differentiation of dendrites in the cerebellar cortex was described (6). Here, we uncovered a crucial function of the transcription factor MEF2C in the homeostatic control of activity-dependent synaptogenesis that could play a role in the establishment of functional neuronal circuits during development and memory storage.
Early in development, many synapses are established in the absence of activity and activity-dependent release (25, 26). At later developmental stages, synaptogenesis takes place under conditions of activity-dependent refinement of connectivity between neurons. In the adult brain, the configuration of synaptic connections is influenced by extensive experience-dependent plasticity. In addition, there is evidence for activity-dependent synaptogenesis in the adult cortex in response to sensory stimulation. Studies of learning and memory have shown that long-term changes in synaptic strength in the hippocampus are accompanied by structural rearrangements, through the emergence of new dendritic spines, which likely corresponds to the formation of new synapses. This activity-dependent increase in spine density could underlie Hebbian plasticity during development and memory storage (27, 28).
Here, we show that developmental deletion of Mef2c in the forebrain causes hippocampus-dependent learning and memory impairment. By contrast, a hippocampus-independent form of learning was unaffected by this genetic manipulation. Surprisingly, accompanying the profound deficit in learning and memory was a dramatic increase in the number of excitatory synapses and potentiation of both basal and evoked synaptic transmission of the perforant path. Conversely, transgenic expression of a superactive form of MEF2C in differentiated dentate granule neurons resulted in a decreased number of excitatory postsynaptic sites without affecting presynaptic properties or learning and memory performance. Interestingly, the dissociated primary cell culture of Mef2c mutant hippocampus revealed that lack of MEF2C does not affect synaptogenesis de novo or initial assembly of synapses. These results indicate that in vivo MEF2C is required to limit excessive increases in the number of excitatory synapses, although it does not hinder synaptogenesis seen during initial development of synaptic connectivity.
MEF2C protein is detected at both presynaptic (entorhinal cortex) and postsynaptic (dentate gyrus granule neurons) sites of the perforant path. Our results provide genetic evidence of a role for MEF2C at both synaptic terminals. When MEF2C is deleted at both presynaptic and postsynaptic neurons of the perforant path, the combined loss results in increased dendritic spine density, increased mEPSCs frequency, and increase in probability of release at the presynaptic site. Expression of a superactivating form of MEF2C only in the postsynaptic cells resulted in decreased dendritic spine density and decreased mEPSCs frequency without affecting presynaptic properties. These findings indicate a cell autonomous role for MEF2C at both synaptic terminals during activity-dependent refinement of synaptic connectivity. It is noteworthy that the functional changes assessed with physiology, e.g., alteration in mEPSCs frequency, may originate from two sources: a change in the number of synapses formed onto a cell and a modification in the release propensity of each synapse. A large fraction of the changes observed in the frequency of mEPSCs is attributable to parallel changes in the number of spines, therefore excitatory synapses; however, the changes in mEPSCs frequency are more robust than the increase in dendritic spine density, thus indicating a significant role for MEF2C activity in regulation of neurotransmitter release probability, which may account for the remaining portion of the alterations in excitatory synaptic function.
Taken together with the activity-dependent effect of MEF2C on transcription of target genes (5), these findings suggest that MEF2C facilitates context-dependent fear conditioning that is a salient aspect of hippocampus-dependent learning and memory, by acting as an activity-dependent negative feedback regulator of increases in synaptic input. Loss of MEF2C leads to impairment of homeostatic regulation of synapse numbers and impairs fear conditioning, whereas MEF2C gain of function limits synapse number and function thus preserving the homeostatic response and the network stability.
Several other transcription factors have been shown to regulate synapse number and the efficiency of synaptic transmission, but the functions of MEF2 in these processes appear to be distinct. The cAMP response element-binding protein (CREB) plays important roles in the conversion of short- to long-term memory (13, 29, 30) and positively influences synapse formation (31, 32). In contrast to the positive impact of the CREB pathway on synaptic inputs, MEF2C keeps synapse number and function under control. These findings suggest a key role for MEF2 transcription factors in counteracting excessive increases in excitatory synaptic drive, thus maintaining a dynamic operating range for regulation of synaptic input into neurons. The absence of the MEF2-mediated control of this synaptic balance leads to an excess of synaptic inputs to target neurons, which may limit their potential for further activity-induced synaptogenesis and structural plasticity (33). Therefore, these results uncover a critical transcriptional pathway, mediated by MEF2C, which homeostatically balances synaptic inputs to target neurons and maintains their sensitivity to respond to environmental stimuli over the long term.
Methods
Generation of MEF2C Mutant and Transgenic Mice.
Details of the generation of mutant and transgenic mice and mouse breeding schemes are described in SI Materials and Methods. As control littermate animals for all of the experiments using Mef2cBKO/KO mice, we used mice carrying the floxed Mef2c allele, which express similar levels of Mef2c mRNA as WT mice.
Tissue Processing, Histology, Immunohistochemistry, RT-PCR, RNA in Situ Hybridization, and TUNEL.
Details of tissue processing, histology, immunohistochemistry, RT-PCR, RNA in situ hybridization, and TUNEL are described in SI Materials and Methods.
Electron Microscopy.
Electron microscopy procedures were performed as described in ref. 34 and in SI Materials and Methods.
Quantification of Dendritic Spine Density.
The procedure to quantify dendritic spine density is based on a previously described method (9) with modifications described in SI Materials and Methods. Images of dendrites within the dentate gyrus area were made by using a ×63 objective with a Leica DM2000 microscope and an Optronics Microfire CCD camera. Spines were counted along dentate granule secondary dendrites starting from their point of origin from the primary dendrite. Using ImageJ software, a dendritic region that was clearly in focus was traced to measure its length. The number of spines on that region was then manually quantified by a blinded counter and divided by the region length to obtain spine density. No correction was made for spines out of focus in the z-plane.
Neuronal Cell Culture.
Whole hippocampi were dissected from the brains of 1-day-old Mef2cBKO/KO mice (n = 5) or littermate controls (n = 7), and dissociated cultures were prepared according to published protocols (35). Immunocytochemistry was performed on cultures 16 days in vitro as described in SI Materials and Methods.
Behavioral Studies.
Locomotor activity, rotarod, elevated plus-maze test, open-field test, fear conditioning, and the pain-sensitivity test were performed as described in ref. 36 on adult male mice at least 2 months old, with littermates of both genotypes. A brief description of each behavior test is provided in SI Materials and Methods.
Electrophysiology.
Hippocampal slices were prepared from 12- to 21-day-old Mef2cBKO/KO and WT littermates or NSE-MEF2C-VP16 transgenic and WT littermate mice (six animals of each genotype). Synaptic activity was recorded from dentate gyrus granule cells (n = 23 for WT and n = 20 for Mef2cBKO/KO; n = 24 for WT and n = 26 for NSE-MEF2C-VP16 transgenic mice) by a whole-cell voltage–clamp technique as described in ref. 35. Description of electrophysiology is provided in the SI Materials and Methods. Recordings were obtained with an Axopatch-200B patch–clamp amplifier (Molecular Devices).
Statistical Analysis.
All data in bar graphs were tested for statistical significance by means of a two-tailed Student's t test.
Supplementary Material
Acknowledgments.
We thank Jonathan Guevara and Xiumin Li for technical assistance, M. Waseem Akhtar for help with experiments, E. J. Kim and John M. Shelton for microscopy assistance, Wei Zhang for assistance with Golgi staining, Dr. Xinran Liu for EM advice, and Dr. Luis Parada (University of Texas Southwestern Medical Center, Dallas) for the GFAP-Cre mice. This work was supported by grants from the National Institutes of Health (National Institute of Mental Health for L.M.M. and E.T.K., and National Heart, Lung, and Blood Institute for E.N.O.), the American Heart Association (E.T.K.), and the Robert A. Welch Foundation (E.N.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802679105/DCSupplemental.
References
- 1.Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of atf4 (creb-2) and c/ebp proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 2.Potthoff MJ, Olson EN. Mef2: A central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 3.Leifer D, Golden J, Kowall NW. Myocyte-specific enhancer binding factor 2c expression in human brain development. Neuroscience. 1994;63:1067–1079. doi: 10.1016/0306-4522(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 4.Lyons GE, Micales BK, Schwarz J, Martin JF, Olson EN. Expression of mef2 genes in the mouse central nervous system suggests a role in neuronal maturation. J Neurosci. 1995;15:5727–5738. doi: 10.1523/JNEUROSCI.15-08-05727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flavell SW, et al. Activity-dependent regulation of mef2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 6.Shalizi A, et al. A calcium-regulated mef2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- 7.Arnold MA, et al. Mef2c transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell. 2007;12:377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor mef2c. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luikart BW, et al. Trkb has a cell-autonomous role in the establishment of hippocampal schaffer collateral synapses. J Neurosci. 2005;25:3774–3786. doi: 10.1523/JNEUROSCI.0041-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malatesta P, et al. Neuronal or glial progeny: Regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhuo L, et al. Hgfap-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 12.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel T, et al. Genetic demonstration of a role for pka in the late phase of ltp and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 14.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 15.Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21(RC135) doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor mef2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto S, Krainc D, Sherman K, Lipton SA. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci USA. 2000;97:7561–7566. doi: 10.1073/pnas.130502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller BU, Konnerth A, Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol. 1998;79:2013–2024. doi: 10.1152/jn.1998.79.4.2013. [DOI] [PubMed] [Google Scholar]
- 20.Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. J Neurosci. 1999;19:6427–6438. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 22.Kwon CH, et al. Neuron-specific enolase-cre mouse line with cre activity in specific neuronal populations. Genesis. 2006;44:130–135. doi: 10.1002/gene.20197. [DOI] [PubMed] [Google Scholar]
- 23.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 24.McKinsey TA, Zhang CL, Olson EN. Mef2: A calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci. 2002;27:40–47. doi: 10.1016/s0968-0004(01)02031-x. [DOI] [PubMed] [Google Scholar]
- 25.Mammen AL, Huganir RL, O'Brien RJ. Redistribution and stabilization of cell surface glutamate receptors during synapse formation. J Neurosci. 1997;17:7351–7358. doi: 10.1523/JNEUROSCI.17-19-07351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 27.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 28.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in ca1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan MP, Abel T. Genetic approaches to the study of synaptic plasticity and memory storage. CNS Spectr. 2003;8:597–610. doi: 10.1017/s1092852900018873. [DOI] [PubMed] [Google Scholar]
- 30.Lonze BE, Ginty DD. Function and regulation of creb family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 31.Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of camkiv and creb. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via cam kinase iv and creb-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 33.Zito K, Svoboda K. Activity-dependent synaptogenesis in the adult mammalian cortex. Neuron. 2002;35:1015–1017. doi: 10.1016/s0896-6273(02)00903-0. [DOI] [PubMed] [Google Scholar]
- 34.Biederer T, et al. Syncam, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 35.Kavalali ET, Klingauf J, Tsien RW. Activity-dependent regulation of synaptic clustering in a hippocampal culture system. Proc Natl Acad Sci USA. 1999;96:12893–12900. doi: 10.1073/pnas.96.22.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gemelli T, et al. Postnatal loss of methyl-cpg binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of rett syndrome in mice. Biol Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.