Abstract
We characterize a sonic hedgehog (Shh) signaling domain restricted to the adventitial layer of artery wall that supports resident Sca1-positive vascular progenitor cells (AdvSca1). Using patched-1 (Ptc1lacZ) and patched-2 (Ptc2lacZ) reporter mice, adventitial Shh signaling activity was first detected at embryonic day (E) 15.5, reached the highest levels between postnatal day 1 (P1) and P10, was diminished in adult vessels, and colocalized with a circumferential ring of Shh protein deposited between the media and adventitia. In Shh−/− mice, AdvSca1 cells normally found at the aortic root were either absent or greatly diminished in number. Using a Wnt1-cre lineage marker that identifies cells of neural crest origin, we found that neither the adventitia nor AdvSca1 cells were labeled in arteries composed of neural crest-derived smooth muscle cells (SMCs). Although AdvSca1 cells do not express SMC marker proteins in vivo, they do express transcription factors thought to be required for SMC differentiation, including serum response factor (SRF) and myocardin family members, and readily differentiate to SMC-like cells in vitro. However, AdvSca1 cells also express potent repressors of SRF-dependent transcription, including Klf4, Msx1, and FoxO4, which may be critical for maintenance of the SMC progenitor phenotype of AdvSca1 cells in vivo. We conclude that a restricted domain of Shh signaling is localized to the arterial adventitia and may play important roles in maintenance of resident vascular SMC progenitor cells in the artery wall.
Keywords: artery, differentiation, Klf4, self-renewal, serum response factor
An adventitia surrounds most blood vessels where it functions as a dynamic compartment for cell trafficking into and out of the artery wall. The adventitia is a site for formation and regression of microvessels that penetrate and nourish the medial and intimal layers of vessel wall (1–3). The adventitia also contains perivascular nerves and lymphatic vessels and is an important domain for NAD(P)H oxidase activity in the vessel wall (4). Adventitial cells participate in vascular growth and repair and are important determinants of lumen size via control of inward or outward wall remodeling processes (2, 5). Recent studies report unexpected roles for the adventitia insofar as it supports resident progenitor cells that can adopt both vascular and nonvascular fates (6–8). Despite these important functional properties, the signals and environmental cues that confer such unique and dynamic properties to the adventitial layer remain largely unknown.
Sonic hedgehog (Shh) is an essential morphogen and growth factor for many developing tissues (9). In the vascular system, Shh signaling is important for specification of arterial-venous identity of endothelial cells (10), remodeling of yolk sac blood vessels (11), and recruitment of mural cells (12). In adult blood vessels, Shh is angiogenic in ischemic hindlimb and corneal micropocket assays (13, 14), promotes perineural neovascularization in diabetic animals (15), and protects the myocardium against chronic ischemia (16). Shh stimulates production of angiogenic factors, including VEGF-A and angiopoietin-1 by interstitial fibroblasts (13, 14), and promotes endothelial cell chemotaxis and tube formation (17, 18). Therefore, Shh plays important roles in cell–cell communication during vascular development and adult wound repair.
Hedgehog (Hh) proteins signal by binding to patched-1 (Ptc1) or patched-2 (Ptc2) receptors and cdo/boc accessory proteins at the cell surface, resulting in derepression of smoothened (smo) (19). Ptc proteins are 12-transmembrane domain receptors that are structurally related to the resistance-nodulation-division (RND) family of bacterial membrane permeases (20) and the Niemann-Pick C1-like1 cholesterol transporter (21). Ptc proteins contain a conserved sterol-sensing domain, and recent studies suggest that Ptc1 represses smo by transporting activating sterols out of the cell (22). Hh binding to Ptc1 inhibits transporter activity thereby allowing activating sterols to accumulate (19, 22). Activated smo trafficks into the primary cilium where Hh signal mediators are concentrated (23, 24) and triggers phosphorylation of gli factors that then move to the nucleus and stimulate gene transcription (25). Among Hh target genes are Ptc1 and Ptc2; therefore, Ptc1lacZ and Ptc2lacZ activities are used as sensitive reporters of Hh signaling in transgenic mice (26, 27).
The origins and functions of the adventitia are poorly understood. Hu et al. (6) reported that stem cell antigen-1 (Sca1)-positive cells with a potential to differentiate into smooth muscle cells (SMCs) were found in the aortic adventitia of adult ApoE−/− mice (AdvSca1 cells). When transferred to the adventitial side of experimental vein grafts, AdvSca1-derived SMCs made up 30% of cells in the graft neointima after 4 weeks (6). The adventitia responds to balloon catheter injury with rapid increases in SMαActin and SM22α expression, particularly at the media–adventitia interface (5). Endothelial progenitor cells are reported to cluster between the media and adventitia in human arteries (7, 8). These cells formed capillary sprouts in ex vivo ring culture assays and were recruited by tumor cells for capillary vessel formation in vivo. Given the role of Shh signaling in the maintenance of resident stem and progenitor cells in skin (28), nervous system (29), lymphoid tissue (30), and hematopoietic cells (31), we sought to test for similar roles for Hh signaling in the adventitial tissue surrounding blood vessels.
Results
Hh Signaling in Postnatal Blood Vessels.
We examined β-gal activity in blood vessels of PtclacZ transgenic mice at postnatal day 2 (P2). In whole-mount hearts, Ptc1lacZ and Ptc2lacZ activities were detected in coronary arteries, but not in myocardium or epicardium (Fig. 1A). The ascending aorta and pulmonary trunk were strongly positive at this time (Fig. 1A). Both Ptc1lacZ and Ptc2lacZ activities declined with age and were detected at low levels in coronary arteries and transverse aorta from adult mice (Fig. 1B). In aortic cross-sections at P2, strong Ptc1lacZ activity was restricted to the adventitia, with only an occasional positive cell found in the media or intima (Fig. 1C). Adventitial Ptc1lacZ activity was also found in intercostal (Fig. 1D) and femoral (Fig. 1F) arteries. In the mesenteric arcade, Ptc1lacZ staining of arteries was more intense than that of veins (Fig. 1E), whereas lymphatic vessels were negative [supporting information (SI) Fig. S1]. Ptc2lacZ activity exhibited very similar patterns (Fig. 1 D–F Right and Fig. S2). Thus, active Hh signaling was found in large and medium-sized arteries and veins in the perinatal period and was localized to the adventitia of these vessels.
Fig. 1.
Hh signaling in artery wall. (A, B, and D–F) Tissues were isolated from PtclacZ mice at P2 and stained for β-gal activity. All major arteries examined, including coronary arteries, aorta, pulmonary trunk (A), intercostal (D), mesenteric (E), and femoral arteries (F) were positive for Ptc1lacZ or Ptc2lacZ activity as shown. (B) Adult hearts displayed low levels of β-gal activity in coronary arteries, aorta, and pulmonary artery. (C) A cross-section of the aorta at P2 reveals Ptc1lacZ-positive cells are restricted to the adventitia. Ica, intercostal artery; A, artery; V, vein; N, nerve. (Magnification: A and D–F, ×7; B, ×3; C, × 40.)
Shh in the Adventitia.
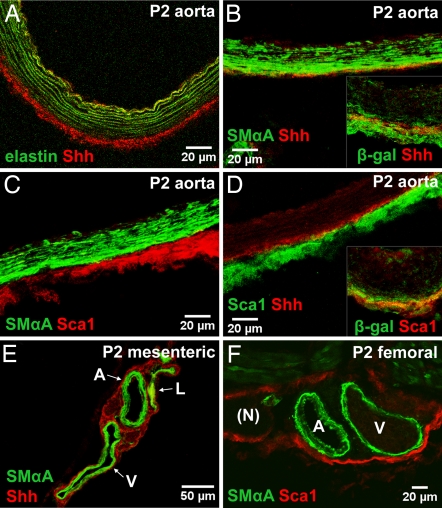
Shh protein was localized to the interface between the media and adventitia in large and medium-sized arteries 2 days after birth (Fig. 2A). This pattern of Shh distribution colocalized with both Ptc1lacZ (Figs. 1C and 2B Inset) and Ptc2lacZ activities. We could not detect Indian hedgehog (Ihh) in postnatal arteries and found only low levels of desert hedgehog (Dhh) in the endothelium (data not shown). These results indicate that Ptc1lacZ and Ptc2lacZ activities in the artery wall correlate with localized deposition of Shh protein in a circumferential ring in the extracellular matrix (ECM)-rich space between the media and adventitia (Fig. 2A).
Fig. 2.
Distribution of Shh and Sca1+ cells in the adventitia. (A) Shh protein is found outside the aortic external elastic lamina (marked by green autofluorescence) at P2. (B) Shh protein (red) is found between the media (SMαActin, green) and adventitia of descending aorta. (B Inset) Shh (red) is colocalized with β-gal in aortic sections from Ptc1lacZ mice. (C) Sca1+ cells (red) reside outside the media (SMαActin, green) in P2 aorta. (D) Sca1+ cells (green) are found in close proximity to Shh (red) and are embedded within a β-gal-positive domain (Inset) in aortic adventitia from Ptc1lacZ mice. (E) Mesenteric vessels stained for SMαActin (green) and Shh (red). (F) Femoral arteries stained for SMαActin (green) and Sca1 (red). Images are stacked Z-plane sections from confocal microscopy. Adventitial localization of Sca1+ cells correlates with the distribution of Shh protein and active Shh signaling in vivo.
Sca1-Positive Cells in the Adventitia.
A previous report (6) described Sca1+ cells in the adventitia surrounding the aortic root in adult ApoE−/− mice. One possible role of Shh may therefore be to maintain Sca1+ progenitor cells within this domain of artery wall. As shown in Fig. 2C, Sca1+ cells are restricted to the adventitia (AdvSca1 cells) and exhibit little or no overlap with SMαActin-positive cells in the media. A similar distribution was found in mesenteric and femoral arteries (Fig. 2 E and F). Close proximity was found between Shh protein and AdvSca1 cells (Fig. 2D). Extensive overlap was observed between β-gal-positive cells and AdvSca1 cells in the aortic adventitia of Ptc1lacZ mice (Fig. 2D Inset). These findings suggest that a zone of Shh signaling in the adventitia identifies a unique domain of artery wall within which resident Sca1+ vascular progenitor cells are found in vivo.
Analysis of Isolated AdvSca1 Cells.
AdvSca1 cells were isolated by immunoselection and examined for progenitor cell markers. AdvSca1 cells exhibited a CD34+/c-kit−/CD140b+ marker profile (Fig. 3A) and were negative for CD45 and CD68 (data not shown), similar to the profile reported by Hu et al. (6). Freshly isolated AdvSca1 cells consistently expressed markers of Shh signaling including ptc1, ptc2, smo, gli1, gli2, and gli3 (Fig. 3A). The distribution of Shh in the extracellular space is governed by multiple binding and transport proteins including hedgehog interacting protein-1 (Hhip1), cdo, and boc (reviewed in ref. 19), all of which are expressed by AdvSca1 cells (Fig. 3A). In addition, AdvSca1 cells contain mRNA for Shh, with few, if any, transcripts detectable for Dhh or Ihh (Fig. 3A). These results suggest that AdvSca1 cells both produce and respond to Shh, and that the restricted distribution of Shh in the artery wall is, in part, locally controlled by factors produced by AdvSca1 cells themselves.
Fig. 3.
Analysis of AdvSca1 cells. (A) RT-PCR analysis of AdvSca1 cells from adult arteries. (B) Freshly isolated AdvSca1 cells (red) contain SRF (green) colocalized with DAPI-stained nuclei (blue) (arrows). (C) Freshly isolated AdvSca1 cells (red) contain Klf4 (green) colocalized with DAPI-stained nuclei (blue) (arrows). (D) RT-PCR analysis of AdvSca1 cells cultured for 0, 12, or 28 days. M is total RNA from aortic media used as a positive control for SMC markers. This analysis was performed three times with similar results. (E) AdvSca1 cells cultured in serum-containing medium for 9 days. Many cells down-regulate ScaI (red) and up-regulate SMαActin (green). (Magnifications: B and C, ×40; E, ×10.)
Hu et al. (6) reported that AdvSca1 cells differentiate into SMCs when incubated with PDGF-BB (6). We found that when cultured in DMEM containing 10% serum many AdvSca1 cells (≈30–50%) lost expression of Sca1, gained expression of SMαActin, SM22α, calponin, and SM-MHC, and adopted an elongated, mesenchymal cell shape (Fig. 3 D and E). To rule out the possibility that up-regulation of SMC markers resulted from expansion of a pool of medial SMCs carried over in the AdvSca1 cell isolation, we repeated our experiments in the presence of inhibitors of cell proliferation (aphidicolin or hydroxyurea). After verifying growth arrest, we observed up-regulation of SMC marker proteins over a similar time course and to a similar extent as seen in the absence of cell cycle inhibitors (Fig. S3). We conclude that AdvSca1 cells can directly differentiate into SMC-like cells, and that this differentiation does not require cell proliferation.
AdvSca1 cells do not express SMC marker proteins in vivo (Figs. 2 and 4). However, they contain mRNAs for transcription factors involved in SMC differentiation, including serum response factor (SRF), myocardin family members, and cysteine-rich LIM proteins Csrp1 and Csrp2 (Fig. 3A). In addition, a subset of freshly isolated AdvSca1 cell nuclei were immunopositive for SRF protein (Fig. 3B). However, AdvSca1 cells also express potent silencers of SRF-dependent transcription, including Msx1, Klf4, and FoxO4 (Fig. 3D and data not shown). Each of these factors can inhibit SRF and myocardin-dependent SMC gene expression (32, 33), and they are down-regulated as AdvSca1 cells acquire SMC markers and increase SRF coactivator expression (Fig. 3D). Therefore, a subset of AdvSca1 cells may be maintained as SMC progenitors by expression of silencers of SRF-dependent transcription. When AdvSca1 cells were incubated with VEGF-A (10 ng/ml, 9 days), PECAM1-positive endothelial cell clusters appeared in elongated cords that often colocalized with SMαActin-positive cells (Fig. S4). When incubated with BMP2 (50 ng/ml, 20 days), colonies formed that stained with alizarin red or von Kossa's stain consistent with differentiation of a subset of AdvSca1 cells to osteogenic cells (Fig. S4). Under conditions described above, 30–50% of AdvSca1 cells differentiated to SMC-like cells, ≈10% produced endothelial cells, and 1–5% formed osteogenic colonies in vitro.
Fig. 4.
AdvSca1 cells in developing aorta. (A–F) Tissues were obtained at the time points indicated. Cross-sections through the aortic root were stained for Sca1 (red), SMαActin (green), and DAPI (blue). AdvSca1 cells first appear between E15.5 and E18.5 in the adventitial space between aorta and pulmonary trunk (white arrows in C) and persist in this location into adulthood. (G) Cross-section from Wnt1-cre/R26R (5 weeks old) aortic root stained for β-gal activity and nuclear fast red. SMCs (blue) but not adventitial cells originate from neural crest. Dashed box is shown at higher magnification in H. (H) Cross-section through aortic root shows no overlap between β-gal-positive and AdvSca1+ cells. Ao, aorta; PT, pulmonary trunk. (Magnification scale bar in E applies to A–F; G, ×10.)
Developmental Origins of AdvSca1 Cells.
Bone marrow reconstitution experiments previously showed that AdvSca1 cells are not marrow-derived (6). To gain insight into the origins of AdvSca1 cells in vivo we examined mice from embryonic day 12.5 (E12.5) to 12 weeks after birth. AdvSca1 cells first appeared in the perivascular space between the ascending aorta and pulmonary trunk between E15.5 and E18.5 (Fig. 4C, arrows), well after the tunica media had acquired SMCs and organized a full complement of elastic lamellae. AdvSca1 cells were present in this position at all subsequent time points examined, and they increased in number with postnatal growth of the artery wall (Fig. 4 D–F). We then used Wnt1-cre transgenic mice to label cells of neural crest origin (34). Wnt1-cre-activated β-gal reporter labeled neural crest-derived SMCs in proximal aorta, pulmonary trunk, common carotid arteries, and ductus arteriosus, but did not label AdvSca1 cells in these vessels (Fig. 4 G and H).
Analysis of AdvSca1 Cells in Shh−/− Embryos.
To determine whether Shh was required for the formation or maintenance of AdvSca1 cells, we examined Shh−/− mice. Two anatomically defined sites were studied. The presence of AdvSca1 cells at the aortic root, where they are first detected during development (Fig. 4C) and are most abundant in adult ApoE−/− mice (6), was confirmed in WT mice (Fig. 5A). By contrast, we could not detect AdvSca1 cells at the aortic root in Shh−/− embryos (Fig. 5B). A small number of AdvSca1 cells were found in the transverse portion of thoracic aorta (Fig. 5 E and F). An absence of septation of the common truncus arteriosus in Shh-deficient embryos has been described (35). AdvSca1 cells were present in the descending thoracic aorta of Shh−/− embryos, but always in reduced numbers (Fig. 5 C–F and Fig. S5). The presence of residual AdvSca1 cells suggests that factors other than Shh are involved in homing and/or maintenance of these cells. Because Dhh expression was detected in the aorta, low levels of Dhh may allow some of these cells to escape a requirement for Shh in the artery wall.
Fig. 5.
AdvSca1 cells in Shh−/− arteries. (A–D) Aortic tissues from WT (A and C) and Shh−/− (B and D) embryos at E18.5. Cross-sections through aortic root (A and B) and descending aorta (C and D) were immunostained for Sca1 (red), SMαActin (green), and DAPI (blue). (E) Solid lines in indicate relative positions of sections in A–D. (F) Dotted line boxes in A–D correspond to aortic segments shown here. AdvSca1 cells are absent in aortic root, greatly reduced in ascending and transverse aorta (termed ascending), and diminished in descending aorta of Shh−/− embryos.
Discussion
We report the characterization of a Shh signaling domain in the adventitial layer of vessel wall. This domain is strongly positive for both Ptc1lacZ and Ptc2lacZ activities and colocalizes with a ring of Shh protein deposited between the media and adventitia. Embedded within this domain is a population of Sca1-expressing vascular progenitor cells, termed AdvSca1 cells, that can differentiate to SMC-like cells ex vivo. The number of AdvSca1 cells in the aortic root is greatly diminished in Shh−/− mice at E18.5. These findings suggest that Shh signaling may play important roles in development of the adventitia and maintenance of a resident population of Sca1+ vascular progenitor cells in the artery wall.
Hh Signaling in Postnatal Artery Wall.
Ptc1lacZ and Ptc2lacZ activities in the adventitia of large and medium-sized arteries were greatest between 1 and 10 days after birth. Although the basic structure of major arteries is established at birth, substantial growth and remodeling occur in the perinatal period. For example, the number of SMCs in rat aorta increases 2.5-fold, wall thickness doubles, and collagen and elastin contents increase >3-fold in the first month after birth (36). These changes in wall structure are adaptations to large perinatal increases in blood flow and blood pressure that are accompanied by corresponding increases in smooth muscle contractile protein mass (37). After 3 months of age, growth of the artery wall is diminished, cell proliferation rates are low, and wall structure has reached an adult state. We found PtclacZ activities and Shh protein levels were maintained at low levels in adult mouse arteries, suggesting that adventitial Shh signaling activity correlates with rapid postnatal growth and remodeling of the artery wall.
Distribution of Shh in the Adventitia.
Ptc1lacZ and Ptc2lacZ activities were colocalized with Shh protein deposited between the media and adventitia. Movement of Hh proteins in the extracellular space is limited by covalent lipid modifications at both amino- and carboxyl-terminal domains (38, 39). Lipid-modified Hh proteins are secreted as multimers that interact with heparan sulfate proteoglycans (HSPGs), which further restricts their movement (40, 41). Our data suggest that Shh protein is concentrated between the media and adventitia because of local synthesis, secretion, and retention of Shh multimers by ECM components, such as the HSPG perlecan (42), in the artery wall. PtclacZ-positive cells are located throughout the adventitia, whether directly adjacent to Shh protein at the media-adventitia interface or several cell layers removed. We do not yet know whether this pattern of PtclacZ activity in vivo is the result of paracrine secretion of Shh by AdvSca1 cells themselves, ECM retention of Shh produced from another source, or some combination of both. However, freshly isolated AdvSca1 contain readily detectable amounts of Shh mRNA, suggesting that local synthesis and secretion of Shh is a normal function of the arterial adventitia.
Analysis of Isolated AdvSca1 Cells.
Embedded within the PtclacZ-positive adventitial domain, we found a population of Sca1-expressing cells that were negative for marker proteins of differentiated SMCs. However, when removed from the adventitia and placed in serum-containing medium, a significant fraction (≈30–50%) of these cells lost Sca1 from the cell surface, acquired a well spread mesenchymal cell shape, and expressed the SMC marker proteins SMαActin, SM22α, calponin, and SM-MHC by 12–28 days. When AdvSca1 cells were exposed to BrdU to label replicating cells, a significant number of Sca1–BrdU double positive cells were found after 7 days, indicating that some AdvSca1 self-renew while, at the same time, others differentiate to SMC-like cells under the same conditions (Fig. S3). These results suggest that AdvSca1 cells are a heterogeneous mixture of cells that can either self-renew or respond to extracellular signals and differentiate to SMC-like cells in vitro.
Maintenance of a Progenitor Phenotype by Transcriptional Corepressors.
AdvSca1 cells do not express SMC marker proteins in vivo. It was intriguing, therefore, to find that AdvSca1 cells contained transcripts for SRF and myocardin family members and were immunopositive for SRF when examined immediately after isolation from the artery wall. These transcription factors are strong activators of SMC gene expression (37, 43–45) and are necessary for vascular SMC differentiation in vivo. Yet SRF also interacts with potent transcriptional corepressors, and it is the competition between coactivators and corepressors that determines whether SRF-dependent target genes are transcribed (44–48). Indeed, we found that the transcriptional corepressors Msx1, Klf4, and FoxO4 were also expressed by AdvSca1 cells. Msx1 forms a ternary complex with SRF and myocardin and inhibits binding of SRF-myocardin to CArG box motifs in SMC target genes (32). Klf4 is a zinc finger-containing protein that binds to a GC-rich element (TCE) and inhibits SRF-dependent transcription of multiple SMC marker genes (47). The forkhead transcription factor FoxO4 interacts with myocardin and inhibits its coactivator function for SRF-dependent SMC gene expression (49). These results are reminiscent of a report by Matsuura et al. (50), showing that Sca1+ cells from adult mouse hearts express Nkx2–5 and GATA4, two transcription factors important for cardiac myocyte differentiation, yet they do not express markers of differentiated myocardial cells. Heart-derived Sca1+ cells differentiate to beating cardiomyocytes when exposed to oxytocin in vitro, thus confirming their cardiogenic potential (50). These findings in heart and artery wall suggest a model in which Sca1+ progenitor cells in postnatal tissues are specified for certain cell fates by expression of transcription factors that are required for those fates. The maintenance of a SMC progenitor phenotype may therefore critically depend on expression of transcriptional corepressors that block SRF-dependent transcription and recruit chromatin remodeling complexes to silence gene expression.
Origins of AdvSca1 Cells.
The origins of AdvSca1 cells are unclear. Hu et al. (6) found no evidence that AdvSca1 cells arise from bone marrow. We investigated this question for aortic AdvSca1 cells by using Wnt1-cre transgenic mice in which β-gal is permanently expressed in neural crest-derived cells (34). AdvSca1 cells directly adjacent to Wnt1-cre-positive aortic SMCs did not express the neural crest marker. Moreover, our developmental time course studies showed that AdvSca1 cells did not appear in aortic arch arteries until between E15.5 and E18.5, much later than the migration of neural crest-derived SMC progenitors into the aortic arch complex ≈E9.5. Two other lineages contribute SMCs to the aorta and other great vessels, i.e., paraxial somitic mesoderm and secondary heart field (51). Lineage-specific cre mice are available for both of these progenitors to test whether AdvSca1 cells arise from either of these sources.
Summary.
We characterize a novel domain of Shh signaling in the arterial adventitia and show that a population of Sca1+ vascular progenitor cells resides within this domain. Because the adventitia lies between the vessel wall and surrounding tissues, progenitor cells within the adventitia could, in principle, contribute to remodeling, repair, or disease processes in either the vessel wall or in neighboring nonvascular tissues. Because essentially all tissues of the body contain blood vessels, the maintenance and fate of adventitial progenitor cells described here and by Hu et al. (6) may be relevant to a wide variety of normal and pathogenic processes. Future work will examine the role of Hh signaling in responses of the adventitia to tissue injuries involving both vessels and surrounding nonvascular cell types.
Materials and Methods
Animals Used.
All protocols were approved by the Institutional Animal Care and Use Committee at the University of North Carolina. Mice used include Ptc1lacZ (Ptctm1Mps) (26), Ptc2lacZ (Ptc2tm1Dgen; Jackson Laboratories; 005827), Wnt1cre (Jackson Laboratories; 003829), Shhtm1Amc (Jackson Laboratories; 003318), Rosa26 (52), CD1 (Charles River), 129/SvEvJ and C57BL/6J (Jackson Laboratories). Noon on the day of vaginal plug was designated E0.5.
Whole-Mount β-Gal Activity.
Embryos or tissues were fixed in fresh 0.2% glutaraldehyde in 0.1 M sodium phosphate, 5 mM EGTA, 2 mM MgCl2, pH 7.3, for 1 h on ice, washed three times with rinse buffer (10 mM sodium phosphate, 2 mM MgCl2, and 0.1% Triton X-100, pH 7.4) for 30 min each at room temperature, and stained as described in SI Text.
Immunofluorescence Staining.
Tissues were fixed in 4% paraformaldehyde (PFA), rinsed in PBS, saturated with 20% sucrose for cryoprotection, embedded in agar, and frozen in OCT. Cryosections (12 μm) were fixed and stained as described in SI Text. Cultured cells were fixed in 4% PFA for 15 min at room temperature, then stained as described in SI Text. Primary antibodies and imaging methods are described in SI Text.
AdvSca1 Cell Isolation and Culture.
The ascending aorta, aortic arch, and ≈1 cm of the descending aorta were dissected from adult mice and placed in PBS on ice. Endothelium was removed with a cotton swab. Adventitia was dissected and rinsed in HBSS, digested with 14 mg/ml collagenase type 2 (Worthington) and 0.75 mg/ml elastase (Roche) in HBSS for 2 h at 37°C with gentle rocking, and filtered (70 μm). Cells in the filtrate were pelleted at 300 × g then rinsed in PBS + 0.5% BSA. Aortic Sca1+ cells were isolated by using anti-Sca1 immunomagnetic MicroBeads and a MACS cell separation system (Miltenyi). Cells were passed over two consecutive columns to increase purity of the isolation. A typical Sca1+ fraction thus prepared is 1–4% of total cells, with 80–95% of isolated cells positive for Sca1 antigen, and <1% of isolated cells positive for SMαActin by immunostaining. Isolated Sca1+ cells were cultured in DMEM (Sigma) plus 10% FBS (HyClone) and 1× antibiotic/antimycotic solution (Gibco) at 37°C, 5% CO2. Cells were seeded in 48-well tissue culture plates at 8 × 103 cells per well. Growth factors tested were VEGF-A (10 ng/ml; Clonetics) and BMP2 (50 ng/ml, PeproTech).
RT-PCR Analysis.
Total cellular RNA was isolated by guanidinium isothiocyanate denaturation and phenol/chloroform extraction as described (53). Two-step RT-PCR was carried out with the GeneAmp RNA PCR kit (Applied Biosystems) according to the manufacturer's instructions. Primer sequences and PCR conditions are listed in Table S1.
Supplementary Material
Acknowledgments.
We thank Da-Zhi Wang, Frank Conlon, and Jim Faber for helpful discussions. This work was carried out by J.N.P. in partial fulfillment of requirements for the PhD degree in the Curriculum in Genetics and Molecular Biology, University of North Carolina. This work was supported by National Institutes of Health Grants HL-19242 (to M.W.M.), HL-07816 (to C.M.), GM-00851 (to J.N.P.), and HL43174 and HL71993 (to V.L.B.), National Research Service Award F32 HL68484 (to K.H.), American Heart Association Grant 0715320U (to J.N.P. and M.W.M.), and the Carolina Cardiovascular Biology Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711382105/DCSupplemental.
References
- 1.Heistad D, Marcus M, Larsen G, Armstrong M. Role of vasa vasorum in nourishment of the aortic wall. Am J Physiol. 1981;240:H781–H787. doi: 10.1152/ajpheart.1981.240.5.H781. [DOI] [PubMed] [Google Scholar]
- 2.Sartore S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 3.Moulton K, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4726–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haurani M, Pagano P. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: Bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Ryan S, Koteliansky V, Gotwals P, Lindner V. Transforming growth factor-β-dependent events in vascular remodeling following arterial injury. J Vasc Res. 2003;40:37–46. doi: 10.1159/000068937. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zengin E, et al. Vascular wall resident progenitor cells: A source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 8.Pasquinelli G, et al. Thoracic aortas from multi-organ donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 9.McMahon A, Ingham P, Tabin C. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 10.Lawson N, Vogel A, Weinstein B. Sonic hedgehog and vascular endothelial growth factor act upstream of the notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 11.Byrd N, et al. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 12.Nagase M, Natase T, Koshima I, Fujita T. Critical time window of hedgehog-dependent angiogenesis in murine yolk sac. Microvasc Res. 2006;71:85–90. doi: 10.1016/j.mvr.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Pola R, et al. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 14.Pola R, et al. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–485. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- 15.Kusano K, et al. Sonic hedgehog induces arteriogenesis in diabetic vasa nervorum and restores function in diabetic neuropathy. Arterioscler Thromb Vasc Biol. 2004;24:2102–2107. doi: 10.1161/01.ATV.0000144813.44650.75. [DOI] [PubMed] [Google Scholar]
- 16.Kusano K, et al. Sonic hedgehog myocardial gene therapy: Tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2006;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- 17.Kanda S, et al. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278:8244–8249. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- 18.Vokes S, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–4380. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, McMahon A, Allen B. Shifting paradigms in hedgehog signaling. Curr Opin Cell Biol. 2007;19:159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Taipale J, Cooper M, Maiti T, Beachy P. Patched acts catalytically to suppress the activity of smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 21.Incardona J. From sensing cellular sterols to assembling sensory structures. Dev Cell. 2005;8:798–799. doi: 10.1016/j.devcel.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Corcoran R, Scott M. Oxysterols stimulate sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huangfu D, et al. Hedgehog signaling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 24.Corbit K, et al. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 25.Rohatgi R, Milenkovic L, Scott M. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 26.Goodrich L, Milenkovic L, Higgins K, Scott M. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 27.Motoyama J, Takabatake T, Takeshima K, Hui C. Ptch2, a second mouse Patched gene is coexpressed with sonic hedgehog. Nat Genet. 1998;18:104–106. doi: 10.1038/ng0298-104. [DOI] [PubMed] [Google Scholar]
- 28.Adolphe C, et al. An in vivo comparative study of sonic, desert, and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131:5009–5019. doi: 10.1242/dev.01367. [DOI] [PubMed] [Google Scholar]
- 29.Ahn S, Joyner A. In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 30.Uhmann A, et al. The hedgehog receptor patched controls lymphoid lineage commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- 31.Baron M. Embryonic origins of mammalian hematopoiesis. Exp Hematol. 2003;31:1160–1169. doi: 10.1016/j.exphem.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi N, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced Msx1 and Msx2 inhibit myocardin-dependent smooth muscle gene transcription. Mol Cell Biol. 2006;26:9456–9470. doi: 10.1128/MCB.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, et al. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 34.Jiang X, Rowitch D, Soriano P, McMahon A, Sucov H. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- 35.Washington Smoak I, et al. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol. 2005;283:357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Olivetti G, Anversa P, Melissari M, Loud A. Morphometric study of early postnatal development of the thoracic aorta in the rat. Circ Res. 1980;47:417–424. doi: 10.1161/01.res.47.3.417. [DOI] [PubMed] [Google Scholar]
- 37.Owens G, Kumar M, Wamhoff B. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 38.Mann R, Beachy P. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 39.Pepinsky R, et al. Identification of a palmitic acid-modified form of human sonic hedgehog. J Biol Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 40.Zhu A, Scott M. Incredible journey: How do developmental signals travel through tissue? Genes Dev. 2004;18:2985–2997. doi: 10.1101/gad.1233104. [DOI] [PubMed] [Google Scholar]
- 41.Eugster C, Panakova D, Mahmoud A, Eaton S. Lipoprotein-heparan sulfate interactions in the hh pathway. Dev Cell. 2007;13:57–71. doi: 10.1016/j.devcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 42.Carrasco H, Olivares G, Faunes F, Oliva C, Larrain J. Heparan sulfate proteoglycans exert positive and negative effects in Shh activity. J Cell Biochem. 2005;96:831–838. doi: 10.1002/jcb.20586. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Olson E. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Curr Opin Genet Dev. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Miano J, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 45.Chang D, et al. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev Cell. 2003;4:107–118. doi: 10.1016/s1534-5807(02)00396-9. [DOI] [PubMed] [Google Scholar]
- 46.Liu N, Olson E. Coactivator control of cardiovascular growth and remodeling. Curr Opin Cell Biol. 2006;18:715–722. doi: 10.1016/j.ceb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Kawai-Kowase K, Owens G. Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol. 2007;292:C59–C69. doi: 10.1152/ajpcell.00394.2006. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Hu G, Herring BP. Smooth muscle-specific genes are differentially sensitive to inhibition by Elk-1. Mol Cell Biol. 2005;25:9874–9885. doi: 10.1128/MCB.25.22.9874-9885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Wang Z, Yanagisawa H, Olson E. Phenotypic modulation of smooth muscle cells through interaction of FoxO4 and myocardin. Dev Cell. 2005;9:261–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 50.Matsuura K, et al. Adult cardiac sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 51.Majesky M. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 52.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 53.Landerholm T, et al. A role for serum response factor in coronary smooth muscle differentiation from proepicardial cells. Development. 1999;126:2053–2062. doi: 10.1242/dev.126.10.2053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.