Abstract
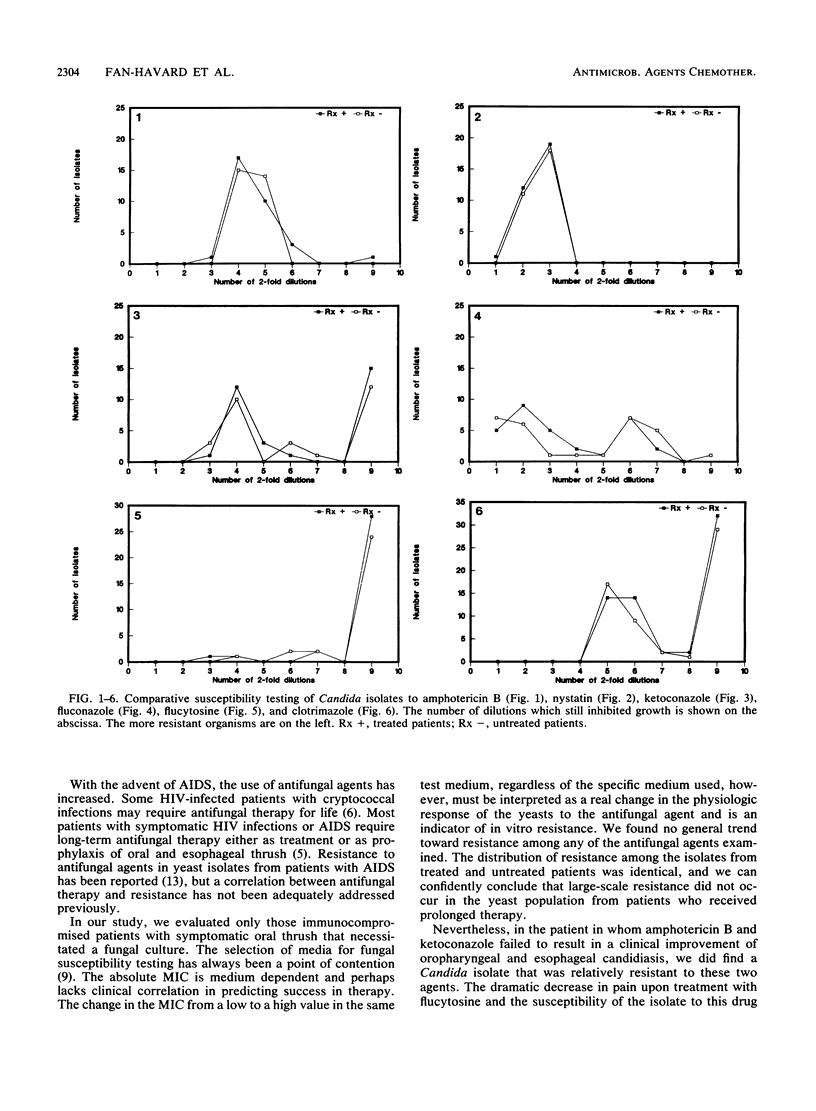
The impact of prolonged antifungal therapy on the development of resistance was examined in 61 patients with oropharyngeal thrush. Fifty-nine patients had symptomatic human immunodeficiency virus infection, one had lung cancer, and one had metastatic prostate cancer. Cultures of pharyngeal samples from all patients were positive for yeasts and included 57 (93.4%) Candida albicans, 3 (4.9%) Candida glabrata, and 1 (1.6%) Candida krusii. Of 61 patients, 32 (52.5%) were receiving or had recently received antifungal therapy. Clotrimazole was the most commonly prescribed azole, followed by ketoconazole and fluconazole. Two patients had received amphotericin B therapy and one had received flucytosine. The duration of therapy with clotrimazole, ketoconazole, and fluconazole ranged from 3 to 240, 14 to 44, and 7 to 138 days, respectively. There was no overall difference in the susceptibilities of the clinical isolates from treated and untreated patients to amphotericin B, nystatin, flucytosine, clotrimazole, ketoconazole, and fluconazole. A.C. albicans isolate from one patient who had clinically failed on ketoconazole, fluconazole, and amphotericin B was resistant to these drugs. The lack of difference in the susceptibility pattern indicates that clinically significant emergence of resistance does not occur in those patients who receive prolonged antifungal therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chachoua A., Dieterich D., Krasinski K., Greene J., Laubenstein L., Wernz J., Buhles W., Koretz S. 9-(1,3-Dihydroxy-2-propoxymethyl)guanine (ganciclovir) in the treatment of cytomegalovirus gastrointestinal disease with the acquired immunodeficiency syndrome. Ann Intern Med. 1987 Aug;107(2):133–137. doi: 10.7326/0003-4819-107-2-133. [DOI] [PubMed] [Google Scholar]
- Defever K. S., Whelan W. L., Rogers A. L., Beneke E. S., Veselenak J. M., Soll D. R. Candida albicans resistance to 5-fluorocytosine: frequency of partially resistant strains among clinical isolates. Antimicrob Agents Chemother. 1982 Nov;22(5):810–815. doi: 10.1128/aac.22.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. D., Merz W. G., Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980 Jul;18(1):158–163. doi: 10.1128/aac.18.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont B., Drouhet E. Fluconazole in the management of oropharyngeal candidosis in a predominantly HIV antibody-positive group of patients. J Med Vet Mycol. 1988 Feb;26(1):67–71. doi: 10.1080/02681218880000081. [DOI] [PubMed] [Google Scholar]
- Eng R. H., Bishburg E., Smith S. M., Kapila R. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am J Med. 1986 Jul;81(1):19–23. doi: 10.1016/0002-9343(86)90176-2. [DOI] [PubMed] [Google Scholar]
- Freedman P. G., Weiner B. C., Balthazar E. J. Cytomegalovirus esophagogastritis in a patient with acquired immunodeficiency syndrome. Am J Gastroenterol. 1985 Jun;80(6):434–437. [PubMed] [Google Scholar]
- Hoeprich P. D., Finn P. D. Obfuscation of the activity of antifungal antimicrobics by culture media. J Infect Dis. 1972 Oct;126(4):353–361. doi: 10.1093/infdis/126.4.353. [DOI] [PubMed] [Google Scholar]
- Kalman C. M., Laskin O. L. Herpes zoster and zosteriform herpes simplex virus infections in immunocompetent adults. Am J Med. 1986 Nov;81(5):775–778. doi: 10.1016/0002-9343(86)90343-8. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Nicholas R. O. Drug resistance in the opportunistic pathogens Candida albicans and Candida glabrata. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):39–49. doi: 10.1093/jac/18.supplement_b.39. [DOI] [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Ollert M., Georgii A., Fröschl M. In vitro susceptibilities and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1988 Dec;26(12):2626–2631. doi: 10.1128/jcm.26.12.2626-2631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983 Nov-Dec;5(6):1033–1048. doi: 10.1093/clinids/5.6.1033. [DOI] [PubMed] [Google Scholar]
- Meunier F. Fluconazole treatment of fungal infections in the immunocompromised host. Semin Oncol. 1990 Jun;17(3 Suppl 6):19–23. [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Bais P., Fan-Havard P., Tecson-Tumang F. Epidemiology of ciprofloxacin resistance among patients with methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1990 Oct;26(4):567–572. doi: 10.1093/jac/26.4.567. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cabezudo I., Wenzel R. P. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):309–317. doi: 10.7326/0003-4819-97-3-309. [DOI] [PubMed] [Google Scholar]



