Abstract
The mammalian inner ear loses its sensory cells with advancing age, accompanied by a functional decrease in balance and hearing. This study investigates oxidant stress in the cochlea of aging male CBA/J mice. Glutathione-conjugated proteins, markers of H2O2-mediated oxidation, began to increase at 12 months of age; 4-hydroxynonenal and 3-nitrotyrosine, products of hydroxyl radical and peroxynitrite action, respectively, were elevated by 18 months. Immunoreactivity to these markers was stronger in the supporting cells (Deiters and pillar cells) than the sensory cells. It appeared later (23 months) in the nerve fibers of the spiral ganglion and in the stria vascularis and spiral ligament. Conversely, antioxidant proteins (AIF) and enzymes (SOD2) decreased by 18 months in the organ of Corti (including the sensory cells) and nerve fibers but not in the stria vascularis. These results suggest the presence of different reactive oxygen species and differential time courses of oxidative changes in individual tissues of the aging cochlea. An imbalance of redox status may be a component of age-related hearing loss.
Keywords: Presbycusis, cochlea, hair cells, lipid peroxidation, protein glutathionylation, nitrotyrosine, superoxide dismutase
1. INTRODUCTION
Studies in a variety of organisms and species support oxidant stress as a factor in the pathology of aging. Levels of reactive oxygen species (ROS) increase in essentially all tissues during aging [7, 27, 28], and mitochondrial DNA deletions, consequences of ROS action, also accumulate progressively [4, 29]. Such biochemical findings are extended by genetic and molecular biological evidence. Mutations in the nematode C. elegans leading to lifespan extension typically also conferred resistance to multiple forms of stress, including oxidant stress [10, 11]. Some of the single-gene mutations that prolonged lifespan in flies [9] also increased stress resistance, and extra copies of genes encoding the antioxidant enzymes superoxide dismutase (SOD) and catalase extended the life span in drosophila [18]. Finally, dietary restriction (reduced caloric intake with nutritional maintenance) enhanced the resistance of the nervous system to age-related diseases by inducing neurotrophic factors and stress proteins that protect neurons against ROS [14].
Oxidative stress may also be part of the aging process in the cochlea. Mitochondrial deletions increase with age and correlate with hearing loss in rats [22]. Furthermore, age-related hearing loss developed earlier and was more severe in mice with genes for SOD deleted [16, 17, 15]. However, little is known about the type of reactive oxygen species that may be involved in the aging process in the inner ear, which tissues and cells (sensory cells and nerve fibers versus supporting structures) are affected and whether the time course of their emergence correlates with the appearance of morphological and functional deficits.
The current study investigates indicators of oxidative stress in the aging cochlea and their distribution in the organ of Corti, the stria vascularis, and the spiral ganglion. The CBA/J mouse was chosen because it carries the ahl-resistant gene [5] thus not rendering it prone to accelerated premature hearing loss as, for example, C57/BL6 or BALB strains. The ages selected for the study represent distinct stages in auditory development and deterioration of the mouse [24]. The auditory system is mature at 3 months of age and has not yet undergone major pathological and pathophysiological changes by 12 months. At 18 months, a moderate hearing loss at high frequencies (24 kHz) can be expected in about 1/3 of the animals, a condition aggravated by 23 months of age when severe hearing loss is seen in 75% of animals. On the cell and tissue level, we studied markers of oxidant stress including glutathione-conjugated proteins, products of H2O2-mediated oxidation, and 4-hydroxynonenal and 3-nitrotyrosine, products of hydroxyl radical and peroxynitrite action. Conversely, antioxidant capacity was followed by analyzing superoxide dismutase and apoptosis-inducing factor histochemically and by Western blotting.
2. MATERIALS AND METHODS
2.1 Materials
ECL (enhanced chemiluminescence) for Western Blotting detection was purchased from Amersham Pharmacia Biotech (Piscataway, NJ); mouse monoclonal antibody for glutathione-conjugated protein from ViroGen (Watertown, MA); monoclonal antibody to 3-nitrotyrosine and polyclonal antibody to 4-hydroxynonenal from Alexis Biochemicals (San Diego, CA); polyclonal anti-manganese superoxide dismutase from Stressgen (Victoria, Canada); polyclonal anti-AIF from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies for Western Blotting were obtained from Jackson Immunoresearch (West Grove, PA); secondary fluorescence antibodies (Alexa 488 and Alexa 546), propidium iodide and Hoechst 33342 from Molecular Probes (Eugene, OR). Complete™ mini EDTA-free protease inhibitor cocktail tablets were purchased from Roche Diagnostic (Mannheim, Germany); Taqman probes from Applied Biosystems (Foster City, CA); BenchMark Protein ladders, TRIzol® reagent and SuperScriptIII First- Strand Synthesis Kit from Invitrogen Life Technologies (Carlsbad, CA); and Master Mixture from Qbiogene (Carlsbad, CA). All other reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
2.2 Animals
Male CBA/J mice were purchased from the National Institute on Aging and acclimated for one week before any experimental procedures. The animals were kept at 22 ± 1°C under a 12h:12h light-dark cycle, and had free access to water and a regular mouse diet (Purina 5025, St. Louis, MO). Animal care was supervised by the University of Michigan’s Unit for Laboratory Animal Medicine and all experimental protocols were approved by the University of Michigan Committee on Use and Care of Animals.
2.3 Western blot analysis
For Western blot analyses, inner ear tissues were first harvested and proteins extracted. Upon sacrifice of the animals, the cochleae were rapidly removed and dissected in ice-cold 10 mM PBS. Combined tissues (organ of Corti, stria vascularis and spiral ligament, and the cochlear portion of the spiral ganglion) from individual cochleae were homogenized in an ice cold RIPA buffer (Upstate, Waltham, MA) by using a micro Tissue Grind pestle for 30 sec. After 15 min on ice, the homogenates were centrifuged at 15,000 × g at 4°C for 10 min and the supernatant fraction was used for further analysis. Protein concentrations were determined by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA).
Protein samples (50 µg) were separated by SDS-PAGE. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Pierce, Rockford, IL) and blocked with 5% nonfat dry milk in PBS-0.1% Tween 20 (PBS-T). The membranes were incubated with anti-SOD2 (1:3,000) or anti-glutathione (1:1,000) antibodies for 2 hr, and then washed 3x (10 min each) with PBS-T buffer. Membranes were incubated with an appropriate secondary antibody at a concentration of 1:10,000 for 1 hr. Following extensive washing of the membrane, the immunoreactive bands were visualized by enhanced chemiluminescence (ECL) on X-ray film according to the instructions of the manufacturer (GE Healthcare, Piscataway, NJ). The membrane was subsequently stained with Imido Black to verify equal loading of protein.
X-ray films of Western blots were scanned and analyzed using AlphaEase software SpotDenso tool (Alpha Innotech, San Leandro, CA). The band densities were first analyzed and normalized to background. Next, the band density of old-age samples was normalized to the control (young animal) samples run on the same gel. Finally, the relative densities of the age groups from four individual assays were averaged and differences tested for statistical significance (p < 0.05) with either a one- or two-tailed one-sample t-test using GraphPad Software (GraphPad Software Inc., San Diego, CA).
2.4 Immunocytochemistry
Immunocytochemical analyses were carried out on cryostat sections. Mice were decapitated and the temporal bones were quickly removed. Cochleae were immediately fixed in 4% paraformaldehyde overnight at 4° C and 8-µm sections were cut on a cryostat. Sections were incubated in 0.5% Triton X-100 for 15 min at room temperature, washed 3x with PBS and blocked with 10% goat serum for 30 min at room temperature, followed by incubation with the primary antibodies at concentrations of 1:500 for anti-4HNE, anti-3 nitrotyrosine, anti-AIF and 1:1,000 for anti-SOD2 at 4°C for 48 hr. After 3 rinses in PBS, the sections were incubated with the secondary antibody (Alexa 488 or Alexa 546 conjugated) at a concentration of 1:500 at 4°C overnight in darkness. After 3 washes with PBS, the sections were incubated with propidium iodide (2 µg/ml in PBS) or Hoechst 33342 (2 µg/ml in PBS) for 30 min at room temperature to stain nuclei. After a final wash with PBS, the slides were mounted. Control incubations were routinely processed without primary antibody. Immunolabeling was visualized and imaged with a Zeiss laser confocal microscope.
2.5 Quantitative RT-PCR (Q-PCR)
For quantitative RT-PCR, cochleae were quickly harvested and dissected in RNAlater® (Ambion, Inc., Austin, TX). Total RNA was isolated from the pooled cochleae of 7 mice by using TRIzol® Reagent and following the manufacturer’s protocol. Total RNA was further purified using RNeasy® Mini Columns following the manufacturer’s protocol for RNA cleanup. Quantity and quality of RNA were determined by RNA 6000 Pico Assay on a Model 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) and spoectrophotometric analysis (Beckman Coulter). RNA was stored at −80°C until analysis.
Total RNA (1 µg) was reverse transcribed to cDNA by using SuperScript III reverse transcriptase (Invitrogen) following the protocol from the manufacturer. The first strand cDNA was diluted (1:10) with DEPC-H2O and stored in aliquots at −20 °C. TaqMan primer and probes were obtained from the ‘Assays by Design’ (SOD2) or ‘Assays on Demand’ services (S16) (ABI, Foster City, CA). A 96-well plate was used for the PCR reactions and triplicate PCR reactions were run for each RNA sample. A housekeeping gene, S16, was also determined on each plate for normalization. The CT value (the number of cycles at which the PCR reaction reaches an arbitrary threshold value) was calculated for each reaction [12].
3. Results
3.1 Oxidative stress increases in the aging cochlea
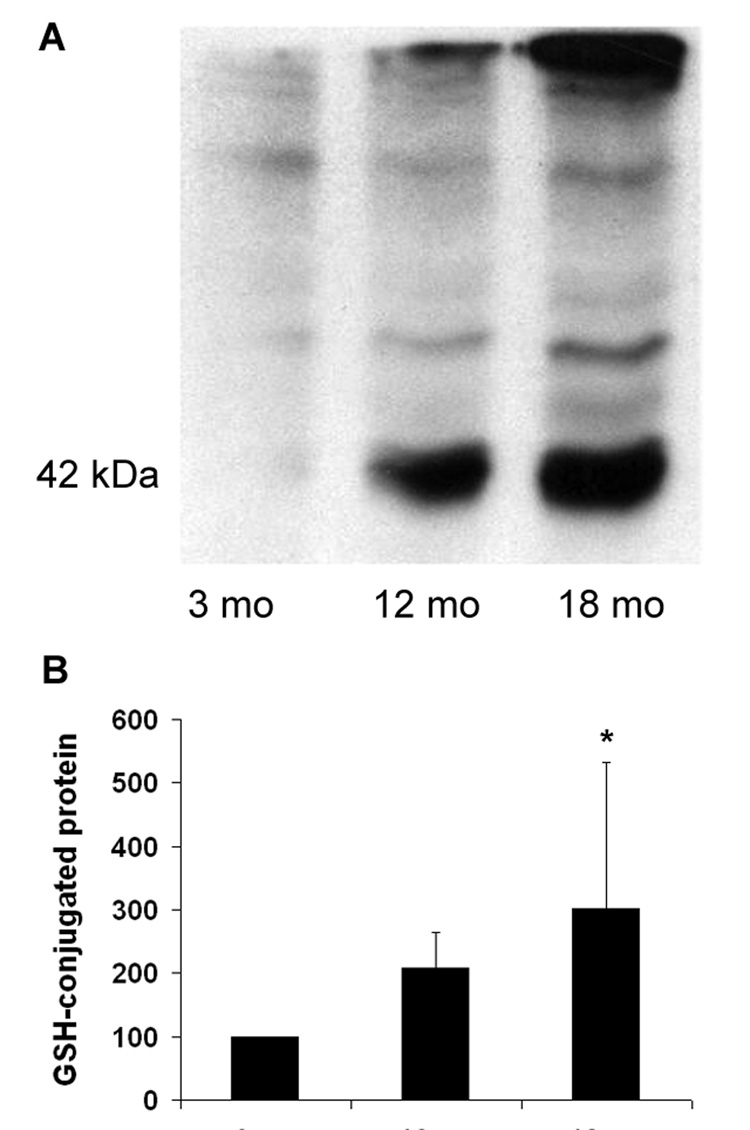
The extent of glutathione-conjugation to proteins is considered a measure of oxidative stress, especially due to H2O2 [30]. Glutathionylated proteins, including a very prominent conjugate with actin at 42 kDa, increased in cochlear extracts with age. Densitometry confirmed a significant 3-fold increase at 18 months compared to young mice (fig. 1).
Figure 1. Glutathione-conjugation to proteins increases with age.
Panel A: Cochlear protein samples were separated by SDS-PAGE on a 10% gel under non-reducing conditions (omission of mercapto ethanol from the PAGE buffer) and Western blots were stained for glutathione as described in “Methods”. Several glutathione-conjugated proteins, including a highly glutathionylated band at 42 kDa, were consistently detected.
Panel B: Quantification of the 42 kDa band as percent of 3-months values.
* The difference between 3 and 18 months is significant (p < 0.05; n = 4).
Next, we used immunohistochemistry to localize 4-hydroxynonenal (4-HNE), a marker for lipid peroxydation [3], and 3-nitrotyrosine (3-NT), a neurochemical marker for oxidation by peroxynitrite [26] at the ages of 3, 12, 18 and 23 months. Staining for both 4-HNE and 3-NT was present in all cell types of the sensory epithelium (organ of Corti) but remained weak at the ages of 3 and 12 months. Staining increased at 18 months and even more so at 23 months, especially in the area of the phalangeal processes of Deiters cells and the inner and outer pillar cells (fig. 2A). In the spiral ganglion cells immunostaining remained weak at ages up to 18 months but increased at 23 months (fig. 2B).
Figure 2. Immunostaining of 4-HNE and 3-NT increases with age.
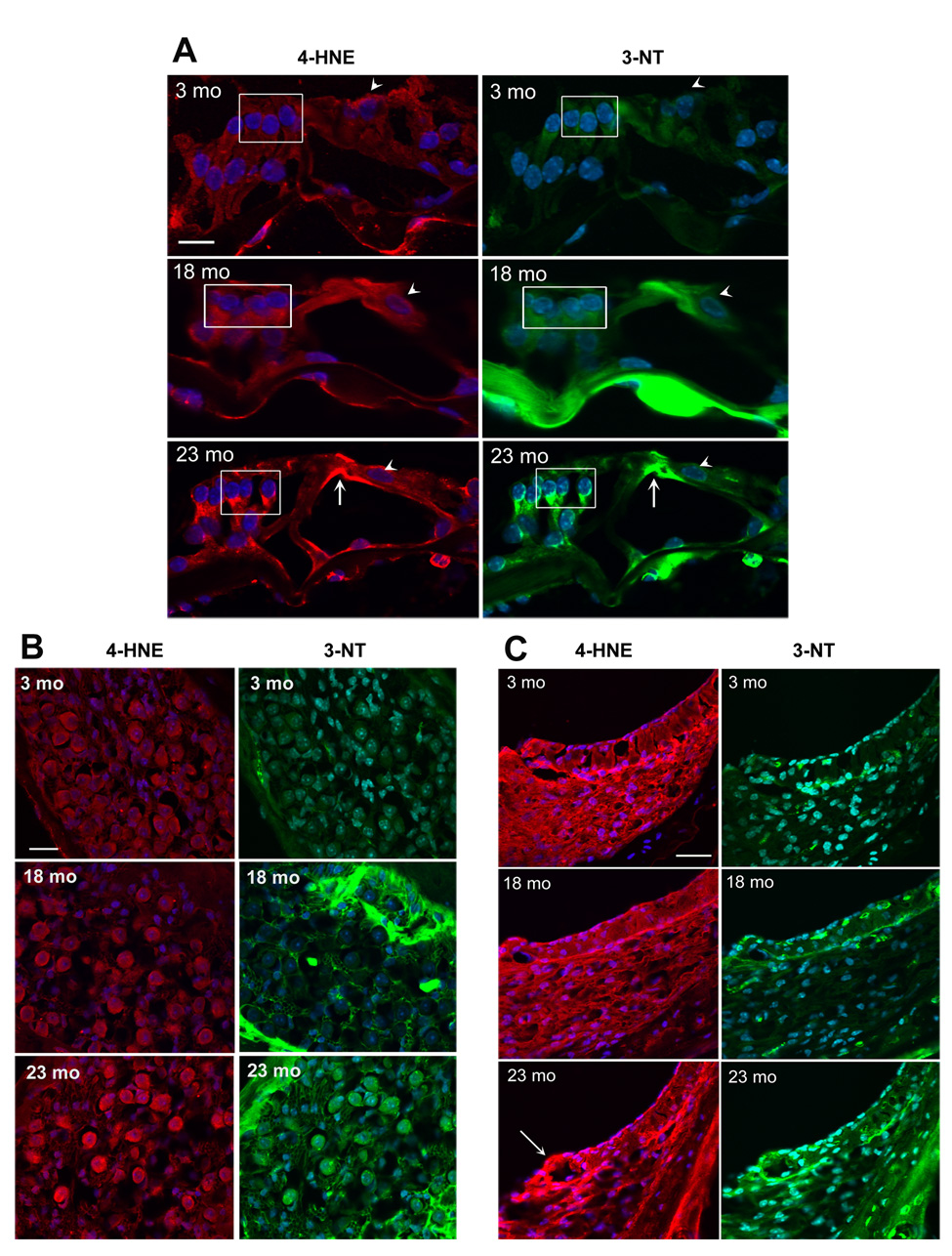
Sections from the basal turn of the cochlea were prepared and stained as described in ‘Methods’. Red fluorescence, 4-HNE; green fluorescence, 3-NT; blue fluorescence, DAPI staining for nuclei. Scale bar: 10 µm.
Panel A: Immunostaining of 4-HNE and 3-NT in the organ of Corti is associated with the phalangeal processes of Deiters cells (box) and the inner and outer pillar cells (arrow). The staining was consistently stronger at 18 and 23 months than at 3 and 12 months (12 months not shown). Staining of inner hair cells was less affected by age (arrow heads). The figures are representative of three animals for each condition.
Panel B: Immunostaining of 4-HNE and 3-NT increased in spiral ganglion cells at the age of 23 months, but not at 18 months. The figures are representative of three animals for each condition. The bright green areas in the 18- and 23-mo sections are bony structures around the ganglion cells.
Panel C: Immunostaining of 4-HNE and 3-NT in stria vascularis increased in specific segments of the lateral wall tissues at the age of 23 months. Mostly affected was the area of the spiral prominence (arrow). At 23 months, the thickness of spiral ligament (fibroblasts and other cell types) appeared reduced. The figures are representative of three animals for each condition.
A similar temporal pattern of staining for both 4-HNE and 3-NT was seen in the stria vascularis, a supporting structure at the lateral wall of the cochlea, responsible for the maintenance of the endocochlear potential (fig. 2C). Staining at 23 months was most intense in the basal layer of the stria vascularis and fibroblasts in the area of the spiral prominence (arrow). A decrease in the width of the spiral ligament was also evident in all sections from 23-months old animals.
3.2 Antioxidants decrease in the aging cochlea
Mitochondrial apoptosis-inducing factor (AIF) is considered a free radical scavenger that is able to prevent apoptosis by a mechanism separate from its function as a transcription factor [6]. The immunostaining for AIF was very strong in the cytosol of all cell types of the organ of Corti at the ages of 3 and 12 months, but it clearly decreased at 18 months. The decrease was primarily evident in outer hair cells and supporting cells (fig. 3).
Figure 3. AIF decreases in the organ of Corti with age.
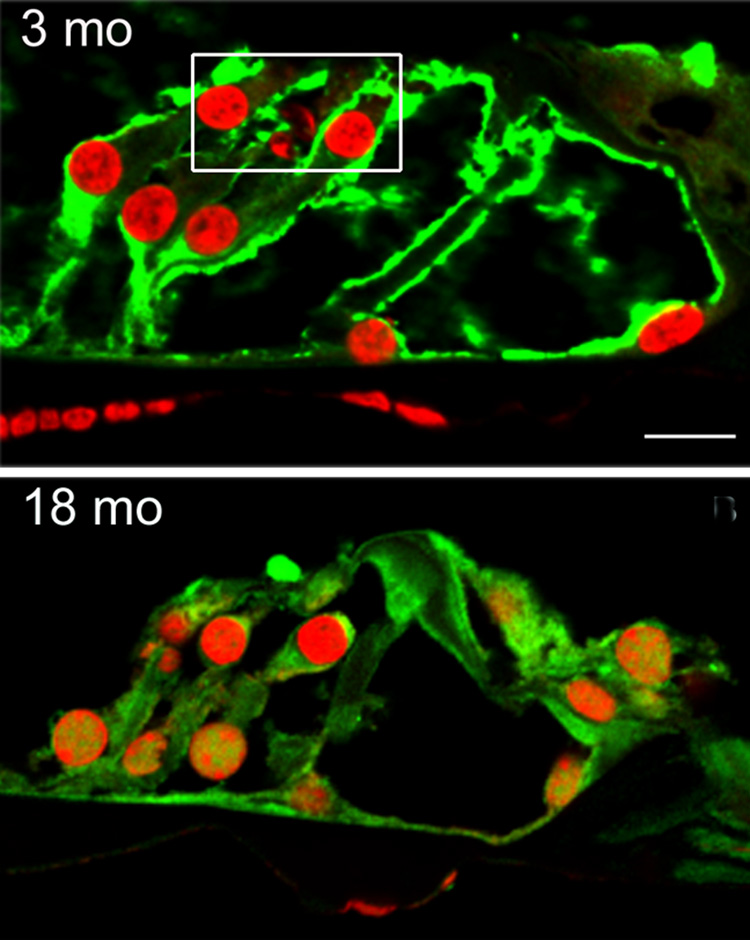
Sections from the basal turn of the cochlea were prepared and stained as described in ‘Methods’. Immunoreactivity to AIF (green) is reduced in the organ of Corti at the age of 18 months compared to sections from 3-months old animals. The AIF staining is decreased in the cytosol of outer hair cells (box) and adjacent Deiters cells, but there is no apparent change in the vicinity of inner hair cells. Red fluorescence is PI staining for nuclei. The figure is representative of three animals for each age. Scale bar: 10 µm.
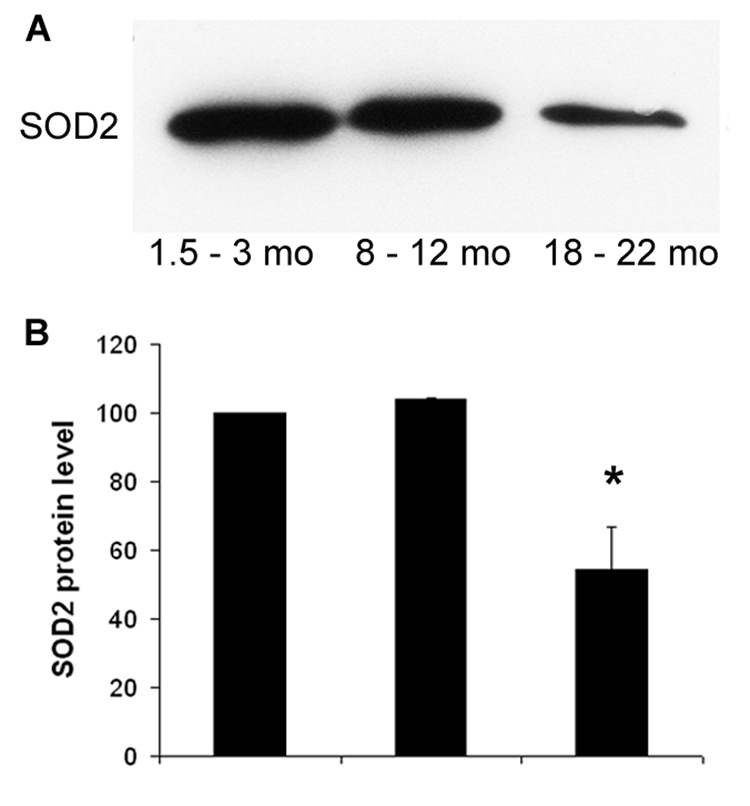
Mitochondrial SOD2 (Mn-SOD) plays a crucial role against oxidative stress since mitochondrial respiration is the major source of reactive oxygen species in the cell. As determined by Western blotting of cochlear extracts, SOD2 decreased about 50% (p < 0.01) at old age compared to young and middle aged mice (fig. 4). Immunostaining for SOD2 in frozen sections of the young organ of Corti indicated the presence of SOD2 in the mitochondria of all cell types. At the age of 18 months, SOD2 decreased in the outer hair cell region (boxed in the 3-mo panel) and the supporting cells. The staining further decreased at 23 months and affected all cell types in the organ of Corti (fig. 5A).
Figure 4. SOD2 decreases in the aging cochleae with age.
Panel A: Cochlear protein samples were separated by SDS-PAGE on a 13% gel and Western blots were processed as described in “Methods”.
Panel B: Densitometry of the gels and statistical analysis confirmed a decrease of 50% in old (18 to 22 months) compared to young (1.5 to 3 months) and middle-aged animals (8 to 12 months). Data are represented as percent of 3-month values.
* P < 0.05; n = 5.
Figure 5. immunoreactivity for SOD2 in the inner ear.
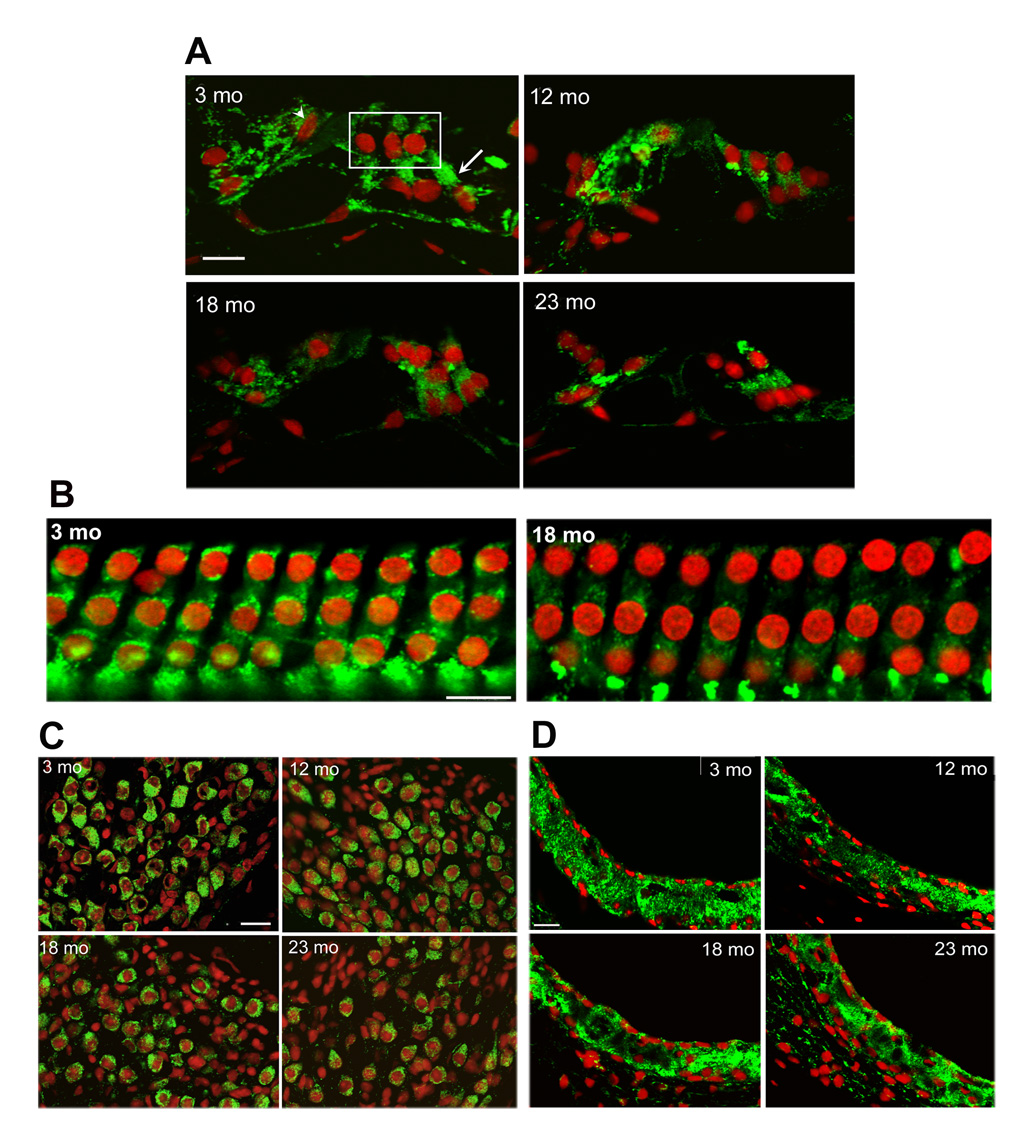
Sections from the basal turn of the cochlea were prepared and stained as described in ‘Methods’. Green fluorescence, SOD2; red fluorescence, PI stain for nuclei. Scale bars: 10 µm.
Panel A: In the organ of Corti, immunostaining localized mitochondrial SOD2 in all cell types of 3-months old animals, including outer hair cells (box), inner hair cells (arrow head) and supporting cells (arrow). Staining decreased at later ages. Panels represent three animals for each condition.
Panel B: Surface preparations from the basal turn of the organ of Corti showed punctate localization of SOD2 in the mitochondria of the outer hair cells at 3 months which decreased at 18 months. Staining for nuclei showed no loss of hair cells. Panels represent three animals for each condition.
Panel C: The staining of SOD2 in the mitochondria of spiral ganglion cells was decreased at the age of 18 months and more so at 23 months. Staining with PI suggests a reduction of the number of cell nuclei at 23 months. Panels are representative of three animals for each condition.
Panel D: No difference in staining intensity was seen in sections of the stria vascularis at different ages. Panels are representative of three animals for each condition.
To probe the SOD2 expression in outer hair cells in more detail, we localized SOD2 in surface preparations. Punctate staining of SOD2, suggesting the association with the mitochondria, was evident at the age of 3 months, and decreased at 18 months (fig. 5B).
In spiral ganglion cells mitochondrial staining for SOD2 began to decrease at 18 months (fig. 5C). In contrast, the staining of SOD2 in the stria vascularis did not change between any of the four age groups (fig 5D). Furthermore, these sections as well as those for AIF staining did not indicate any reduction in the thickness of the stria vascularis.
3.3 The decrease in SOD2 is not due to decreased gene expression
For RT-PCR, mRNA was extracted and pooled from 14 cochleae. The SOD2 mRNA level was measured in aliquots from such pools (3 pools per age group) using a TaqMan Probe. All expression levels were normalized to the housekeeping gene S16 and were calculated relative to the expression at 3 months of age. The relative SOD2 mRNA expression levels showed a trend towards an elevated mRNA-level with age (data not shown), with marginally significant changes of 1.2- and 1.75-fold at the age of 12 and 18 months, respectively (0.1 > p > 0.05).
4. Discussion
An oxidative imbalance is clearly evident in the aging mouse cochlea. Such an imbalance can result from increased production of reactive oxygen species or weakened defense systems, and both mechanisms may participate here. All markers of oxidant stress, lipid peroxidation, glutathionylation and nitrosylation of proteins increase whereas the measures of antioxidant defenses, AIF and SOD2, decrease with age.
While mitochondrial deletions have previously been detected in the aging inner ear [19, 22], we can now show several different mechanisms that may contribute to oxidative stress. The accumulation of glutathionylated proteins is an indicator of protein oxidation arising from the formation of hydrogen peroxide [30, 20, 2]. The rise of 4-HNE signals lipid peroxidation, mainly due to the production of hydroxyl radical, and 3-NT suggests peroxynitrite reactions [26].
SOD2, the mitochondrial enzyme inactivating the superoxide radical and therefore a measure of antioxidant capacity, decreased significantly with age. However, mRNA of SOD2 does not decrease, indicating that the lower amount of SOD2 is not due to decreased gene expression. Several other processes may affect protein levels, an accelerated degradation due to mitochondrial ROS being a likely possibility. This suggestion is in agreement with the observation that mitochondrial proteins are particularly vulnerable to such a mechanism of inactivation in other aging systems [1].
AIF has two distinct functions based on specific binding regions: a putative DNA binding site for its role as transcription factor and a second site which confers oxido-reductase activity [13, 31]. The localization of AIF in the cytoplasm without translocation to the nuclei at any age implies a role as an antioxidant in the organ of Corti. It’s decrease, together with the decrease in SOD2, is a strong indication of compromised antioxidant capacity in the aging cochlea.
In the pathology of sensorineural presbycusis, the loss of hair cells in the organ of Corti is one of the initial manifestations, followed by a degeneration of the auditory nerve. The temporal sequence of increasing oxidative imbalance in inner ear tissues appears to match this pattern well. In the organ of Corti, oxidant stress is apparent by 18 months while staining for the same markers in nerve fibers does not increase until 23 months. Conversely, SOD2 begins to decrease in the organ of Corti, particularly in the outer hair cell area, by 12 months and is further reduced by 18 months and older. In the nerve fibers a significant reduction of SOD2 staining is seen at 18 months and more so at 23 months. Interestingly, the SOD2 protein in the stria vascularis, an accessory structure responsible for maintaining electrolyte balance and electric potential of the endolymphatic fluid, remains unchanged with age, and the integrity of this tissue seems to be preserved. Stria vascularis can show severe morphological alterations in a “metabolic” or “strial” presbycusis but it can retain normal appearance in sensorineural hearing loss [21].
In contrast to the preferential loss of outer hair cells with age, the increased oxidative stress in the organ of Corti is not restricted to the sensory cells. The phalangeal process of Deiters cells and the inner and outer pillar cells show highly elevated levels of stress markers. More than a single cell type is also affected by the decrease in antioxidant capacity. Oxidative imbalance may therefore be a general phenomenon associated with aging while its pathological consequences may be based on differing magnitudes of the imbalance or divergent cell- and tissue-specific capacities to restore a homeostatic balance. The fact that the outer hair cells but not the supporting cells undergo early cell death may best be explained by the intrinsic susceptibility of outer hair cells to oxidative stress [25].
In summary, our data provide evidence that various forms of oxidative stress increase in the aging cochlea, while cellular antioxidant defense systems are reduced. The time course of these changes and the structures affected by them agree well with the pattern of pathology and pathophysiology of the cochlea. Oxidative imbalance may, therefore, contribute to age-related hearing loss. Precise causal relationships, however, remain to be established.
ACKNOWLEDGEMENT
This research was supported by a pilot grant from the American Claude Pepper Center at the University of Michigan, program project grant AG-025164 from the National Institute on Aging, and core grant P30 DC-05188 from the National Institute on Deafness and Other Communication Disorders, NIH.
Non-standard abbreviations used
- 4-HNE
4-hydroxynonenal
- AIF
Apoptosis-inducing factor
- ROS
Reactive oxygen species
- SOD2
Manganese superoxide dismutase (mitochondrial)
Footnotes
DISCLOSURE STATEMENT: The authors have no conflicts of interest.
All experimental protocols were approved by the University of Michigan Committee on Use and Care of Animals and animal care was supervised by the University of Michigan’s Unit for Laboratory Animal Medicine.
References
- 1.Basoah A, Matthews PM, Morten KJ. Rapid rates of newly synthesized mitochondrial protein degradation are significantly affected by the generation of mitochondrial free radicals. FEBS Lett. 2005;579:6511–6517. doi: 10.1016/j.febslet.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Chai YC, Ashraf SS, Rokutan K, Johnston RB, Jr, Thomas JA. S-thiolation of individual human neutrophil proteins including actin by stimulation of the respiratory burst: evidence against a role for glutathione disulfide. Arch Biochem Biophys. 1994;310:273–281. doi: 10.1006/abbi.1994.1167. [DOI] [PubMed] [Google Scholar]
- 3.Comporti M. Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic Res. 1998;28:623–635. doi: 10.3109/10715769809065818. [DOI] [PubMed] [Google Scholar]
- 4.Hattori K, Tanaka M, Sugiyama S, Obayashi T, Ito T, Satake T, Hanaki Y, Asai J, Nagano M, Ozawa T. Age-dependent increase in deleted mitochondrial DNA in the human heart: possible contributory factor to presbycardia. Am Heart J. 1991;121:1735–1742. doi: 10.1016/0002-8703(91)90020-i. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KR, Erway LC, Cook SA, Willott JF, Qing YZ. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 6.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 7.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 8.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 9.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophilia mutant Methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 10.Lithgow GJ. Stress response and aging in Caenorhabditis elegans. Results Probl Cell Differ. 2000;29:131–148. doi: 10.1007/978-3-540-48003-7_7. [DOI] [PubMed] [Google Scholar]
- 11.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 1994;49:B270–B276. doi: 10.1093/geronj/49.6.b270. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Mate MJ, Ortiz-Lombardia M, Boitel B, Haouz A, Tello D, Susin SA, Penninger J, Kroemer G, Alzari PM. The crystal structure of the mouse apoptosis-inducing factor AIF. Nat Struct Biol. 2002;9:442–446. doi: 10.1038/nsb793. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech Ageing Dev. 2001;122:757–778. doi: 10.1016/s0047-6374(01)00226-3. [DOI] [PubMed] [Google Scholar]
- 15.McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–321. [PubMed] [Google Scholar]
- 16.McFadden SL, Ding D, Burkard RF, Jiang H, Reaume AG, Flood DG, Salvi RJ. Cu/Zn SOD deficiency potentiates hearing loss and cochlear pathology in aged 129, CD-1 mice. J Comp Neurol. 1999a;413:101–112. [PubMed] [Google Scholar]
- 17.McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999b;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 18.Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motor neurons. Nature Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 19.Pickles JO. Mutation in mitochondrial DNA as a cause of presbycusis. Audiol Neurootol. 2004;9:23–33. doi: 10.1159/000074184. [DOI] [PubMed] [Google Scholar]
- 20.Rokutan K, Johnston RB, Jr, Kawai K. Oxidative stress induces S-thiolation of specific proteins in cultured gastric mucosal cells. Am J Physiol. 1994;266:G247–G254. doi: 10.1152/ajpgi.1994.266.2.G247. [DOI] [PubMed] [Google Scholar]
- 21.Schuknecht HF. Pathology of the Ear. Cambridge: Harvard University Press; 1974. pp. 388–409. [Google Scholar]
- 22.Seidman MD, Bai U, Khan MJ, Quirk WS. Mitochondrial DNA deletions associated with aging and presbyacusis. Arch Otolaryngol Head Neck Surg. 1997;123:1039–1045. doi: 10.1001/archotol.1997.01900100009001. [DOI] [PubMed] [Google Scholar]
- 23.Seidman MD. Effects of dietary restriction and antioxidants on presbycusis. Laryngoscope. 2000;110:727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Sha SH, Jiang H, Dootz G, Schacht J. Cochlear antioxidant defense systems in presbycusis. Abstr Midwinter Res Meet Assoc Res Otolaryngol. 2004;27:656. [Google Scholar]
- 25.Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 26.Shin CM, Chung YH, Kim MJ, Lee EY, Kim EG, Cha CI. Age-related changes in the distribution of nitrotyrosine in the cerebral cortex and hippocampus of rats. Brain Res. 2002;931:194–199. doi: 10.1016/s0006-8993(01)03391-1. [DOI] [PubMed] [Google Scholar]
- 27.Sohal RS, Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic Biol Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 28.Sohal RS, Weindruch R. Oxidant stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DC. A mitochondrial paradigm for degenerative diseases and ageing. Novartis Found Symp. 2001;235:247–263. doi: 10.1002/0470868694.ch20. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276:47763–47766. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 31.Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N, Daugas E, Garrido C, Kroemer G, Wu H. DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol. 2002;9:680–684. doi: 10.1038/nsb836. [DOI] [PubMed] [Google Scholar]