Abstract
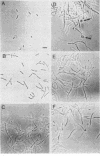
The relative binding affinities of the extended-spectrum cephalosporins cefepime, cefpirome, and cefaclidine for the penicillin-binding proteins (PBPs) of Escherichia coli K-12 and Pseudomonas aeruginosa SC8329 were determined. Affinities were calculated from competition experiments between these antibiotics and [3H]benzylpenicillin in isolated membrane preparations. The concentrations which reduced binding to a PBP by 50% (IC50s) were determined. For E. coli, all three antibiotics displayed good PBP 3 binding (IC50s of 0.5 microgram/ml or less), and MICs roughly correlated with these values. Cefepime had a greater than 20-fold-lower IC50 for PBP 2 of E. coli than the other antibiotics. For P. aeruginosa, all of the antibiotics bound poorly (greater than 25 micrograms/ml) to PBP 2 but showed excellent pseudomonal (less than 0.0025 microgram/ml) PBP 3 binding. No correlations were seen between IC50s and MICs for P. aeruginosa. Despite differences in PBP binding, cefepime, cefpirome, and cefaclidine all displayed similar bactericidal activity for E. coli K-12 over the initial 3 h after antibiotic addition. All three caused E. coli to form filaments at values close to the MICs. In addition, cefepime induced "bleb" formation along the filaments at concentrations greater than 10x the MIC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellido F., Pechère J. C., Hancock R. E. Novel method for measurement of outer membrane permeability to new beta-lactams in intact Enterobacter cloacae cells. Antimicrob Agents Chemother. 1991 Jan;35(1):68–72. doi: 10.1128/aac.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido F., Pechère J. C., Hancock R. E. Reevaluation of the factors involved in the efficacy of new beta-lactams against Enterobacter cloacae. Antimicrob Agents Chemother. 1991 Jan;35(1):73–78. doi: 10.1128/aac.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka M., Inoue M., Mitsuhashi S. Hydrolytic rate at low drug concentration as a limiting factor in resistance to newer cephalosporins. Rev Infect Dis. 1988 Jul-Aug;10(4):746–751. doi: 10.1093/clinids/10.4.746. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Masuyoshi S., Mitsuhashi S., Tomatsu K., Inoue M. Cephalosporinase interactions and antimicrobial activity of BMY-28142, ceftazidime and cefotaxime. J Antibiot (Tokyo) 1988 Jan;41(1):86–93. doi: 10.7164/antibiotics.41.86. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L. beta-Lactamases and beta-lactam resistance in Escherichia coli. Antimicrob Agents Chemother. 1985 Nov;28(5):703–705. doi: 10.1128/aac.28.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Arai S., Hayashi S., Fujimoto K. Beta-lactamase stability of cefpirome (HR 810), a new cephalosporin with a broad antimicrobial spectrum. Antimicrob Agents Chemother. 1986 Nov;30(5):713–718. doi: 10.1128/aac.30.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Permeation of beta-lactam antibiotics into Escherichia coli, Pseudomonas aeruginosa, and other gram-negative bacteria. Rev Infect Dis. 1988 Jul-Aug;10(4):691–698. doi: 10.1093/clinids/10.4.691. [DOI] [PubMed] [Google Scholar]
- Nichols W. W. Towards a fundamental understanding of the MIC of beta-lactam antibiotics. J Antimicrob Chemother. 1988 Sep;22(3):275–283. doi: 10.1093/jac/22.3.275. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Bacterial resistance to antibiotics as a function of outer membrane permeability. J Antimicrob Chemother. 1988 Jul;22 (Suppl A):17–22. doi: 10.1093/jac/22.supplement_a.17. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Liu W., Rosenberg E. Y. Outer membrane permeability and beta-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990 Feb;34(2):337–342. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Phelps D. J., Carlton D. D., Farrell C. A., Kessler R. E. Affinity of cephalosporins for beta-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986 May;29(5):845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis. 1983 Mar;147(3):585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Cromie K. D. Penicillin-binding proteins of gram-negative bacteria. Rev Infect Dis. 1988 Jul-Aug;10(4):699–711. doi: 10.1093/clinids/10.4.699. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- Sumita Y., Fukasawa M., Okuda T. Comparison of two carbapenems, SM-7338 and imipenem: affinities for penicillin-binding proteins and morphological changes. J Antibiot (Tokyo) 1990 Mar;43(3):314–320. doi: 10.7164/antibiotics.43.314. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Katsu K., Moriyama M., Kitoh K. In vitro evaluation of E1040, a new cephalosporin with potent antipseudomonal activity. Antimicrob Agents Chemother. 1988 May;32(5):693–701. doi: 10.1128/aac.32.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]