Abstract
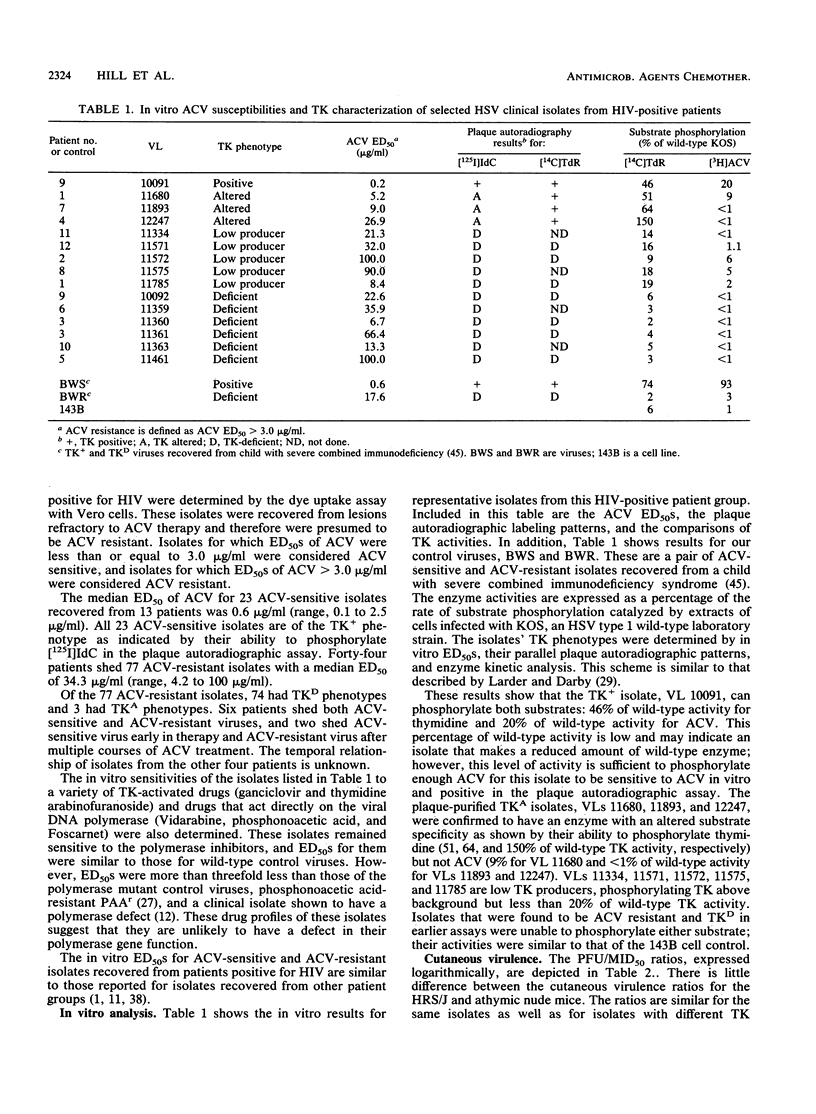
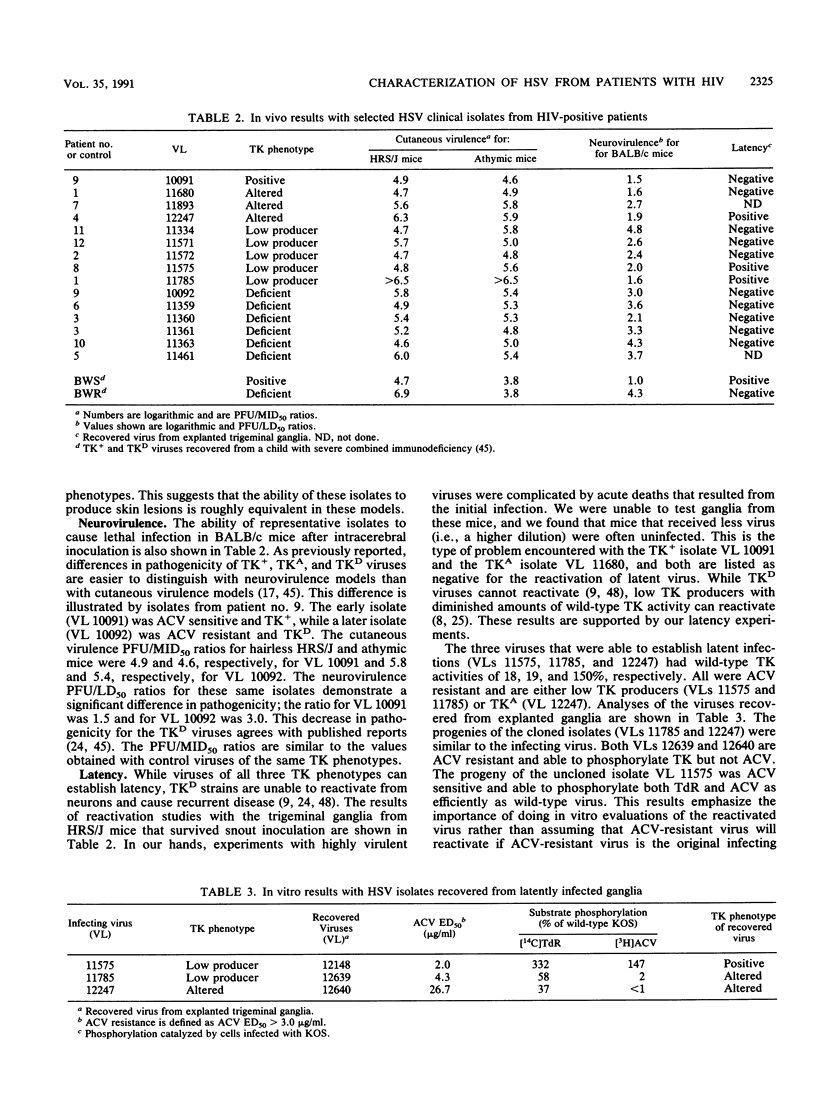
A total of 100 herpes simplex viruses isolated from lesions not responding to acyclovir (ACV) therapy were recovered from 51 patients infected with human immunodeficiency virus. In vitro analysis of these isolates included testing their susceptibility to ACV and determining their thymidine kinase (TK) phenotypes. Of the 100 isolates evaluated, 23 were ACV sensitive and 77 were ACV resistant. Seventy-four of these ACV-resistant isolates were of the TK-deficient or low-TK-producer phenotype and three were of the TK-altered phenotype. The TKs isolates that represented each of the different autoradiographic phenotypes were further characterized by enzyme kinetics. The ability of selected isolates to cause disease in vivo was evaluated by using several mouse virulence models. Cutaneous virulence in normal and immunocompromised mice was evaluated, and neurovirulence in normal mice was determined. Latent infections were assayed by the cocultivation of trigeminal ganglia recovered from mice that had survived acute infection. These reactivated viruses were evaluated in vitro and compared with the original infecting isolate. The mechanisms of resistance and pathogenicity of these herpes simplex virus isolates recovered from patients positive for human immunodeficiency virus are similar to those reported for isolates recovered from normal and immunocompromised patients without AIDS.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry D. W., Lehrman S. N., Ellis M. N. Clinical and laboratory experience with acyclovir-resistant herpes viruses. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):75–84. doi: 10.1093/jac/18.supplement_b.75. [DOI] [PubMed] [Google Scholar]
- Barry D. W., Nusinoff-Lehrman S., Ellis M. N., Biron K. K., Furman P. A. Viral resistance, clinical experience. Scand J Infect Dis Suppl. 1985;47:155–164. [PubMed] [Google Scholar]
- Bean B., Fletcher C., Englund J., Lehrman S. N., Ellis M. N. Progressive mucocutaneous herpes simplex infection due to acyclovir-resistant virus in an immunocompromised patient: correlation of viral susceptibilities and plasma levels with response to therapy. Diagn Microbiol Infect Dis. 1987 Jul;7(3):199–204. doi: 10.1016/0732-8893(87)90005-8. [DOI] [PubMed] [Google Scholar]
- Burns W. H., Saral R., Santos G. W., Laskin O. L., Lietman P. S., McLaren C., Barry D. W. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982 Feb 20;1(8269):421–423. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- Campione-Piccardo J., Rawls W. E., Bacchetti S. Selective assay for herpes simplex viruses expressing thymidine kinase. J Virol. 1979 Aug;31(2):281–287. doi: 10.1128/jvi.31.2.281-287.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Crumpacker C. S. Analysis of the thymidine kinase gene from clinically isolated acyclovir-resistant herpes simplex viruses. Virology. 1991 Feb;180(2):793–797. doi: 10.1016/0042-6822(91)90093-q. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Miller C. H., Schrager L. E., Crumpacker C. S. Successful treatment with foscarnet of an acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):297–300. doi: 10.1056/NEJM198902023200507. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Irmiere A. F., Jacobson J. G., Kerns K. M. Low levels of herpes simplex virus thymidine- thymidylate kinase are not limiting for sensitivity to certain antiviral drugs or for latency in a mouse model. Virology. 1989 Feb;168(2):221–231. doi: 10.1016/0042-6822(89)90261-4. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Larder B. A., Oliver N. M., Kemp S., Smith I. W., Darby G. Characterization of a DNA polymerase mutant of herpes simplex virus from a severely immunocompromised patient receiving acyclovir. J Gen Virol. 1989 Feb;70(Pt 2):375–382. doi: 10.1099/0022-1317-70-2-375. [DOI] [PubMed] [Google Scholar]
- Collins P. Viral sensitivity following the introduction of acyclovir. Am J Med. 1988 Aug 29;85(2A):129–134. [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- Darby G., Field H. J., Salisbury S. A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981 Jan 1;289(5793):81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- Dekker C., Ellis M. N., McLaren C., Hunter G., Rogers J., Barry D. W. Virus resistance in clinical practice. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):137–152. doi: 10.1093/jac/12.suppl_b.137. [DOI] [PubMed] [Google Scholar]
- Dorsky D. I., Crumpacker C. S. Drugs five years later: acyclovir. Ann Intern Med. 1987 Dec;107(6):859–874. doi: 10.7326/0003-4819-107-6-859. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Kemp S., Darby G., Minson A. C. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J Gen Virol. 1989 Apr;70(Pt 4):869–879. doi: 10.1099/0022-1317-70-4-869. [DOI] [PubMed] [Google Scholar]
- Ellis M. N., Keller P. M., Fyfe J. A., Martin J. L., Rooney J. F., Straus S. E., Lehrman S. N., Barry D. W. Clinical isolate of herpes simplex virus type 2 that induces a thymidine kinase with altered substrate specificity. Antimicrob Agents Chemother. 1987 Jul;31(7):1117–1125. doi: 10.1128/aac.31.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. N., Martin J. L., Lobe D. C., Johnsrude J. D., Barry D. W. Induction of acyclovir-resistant mutants of herpes simplex virus type I in athymic nude mice. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):95–101. doi: 10.1093/jac/18.supplement_b.95. [DOI] [PubMed] [Google Scholar]
- Engel J. P., Englund J. A., Fletcher C. V., Hill E. L. Treatment of resistant herpes simplex virus with continuous-infusion acyclovir. JAMA. 1990 Mar 23;263(12):1662–1664. [PubMed] [Google Scholar]
- Englund J. A., Zimmerman M. E., Swierkosz E. M., Goodman J. L., Scholl D. R., Balfour H. H., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990 Mar 15;112(6):416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J. Development of clinical resistance to acyclovir in herpes simplex virus-infected mice receiving oral therapy. Antimicrob Agents Chemother. 1982 May;21(5):744–752. doi: 10.1128/aac.21.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Lay E. Characterization of latent infections in mice inoculated with herpes simplex virus which is clinically resistant to acyclovir. Antiviral Res. 1984 Apr;4(1-2):43–52. doi: 10.1016/0166-3542(84)90024-x. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G. Properties of a novel thymidine kinase induced by an acyclovir-resistant herpes simplex virus type 1 mutant. J Virol. 1982 May;42(2):649–658. doi: 10.1128/jvi.42.2.649-658.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Darby G. Virus drug-resistance: mechanisms and consequences. Antiviral Res. 1984 Apr;4(1-2):1–42. doi: 10.1016/0166-3542(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Lehrman S. N., Douglas J. M., Corey L., Barry D. W. Recurrent genital herpes and suppressive oral acyclovir therapy. Relation between clinical outcome and in-vitro drug sensitivity. Ann Intern Med. 1986 Jun;104(6):786–790. doi: 10.7326/0003-4819-104-6-786. [DOI] [PubMed] [Google Scholar]
- Lehrman S. N., Hill E. L., Rooney J. F., Ellis M. N., Barry D. W., Straus S. E. Extended acyclovir therapy for herpes genitalis: changes in virus sensitivity and strain variation. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):85–94. doi: 10.1093/jac/18.supplement_b.85. [DOI] [PubMed] [Google Scholar]
- Ljungman P., Ellis M. N., Hackman R. C., Shepp D. H., Meyers J. D. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J Infect Dis. 1990 Jul;162(1):244–248. doi: 10.1093/infdis/162.1.244. [DOI] [PubMed] [Google Scholar]
- Marks G. L., Nolan P. E., Erlich K. S., Ellis M. N. Mucocutaneous dissemination of acyclovir-resistant herpes simplex virus in a patient with AIDS. Rev Infect Dis. 1989 May-Jun;11(3):474–476. doi: 10.1093/clinids/11.3.474. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Ellis M. N., Keller P. M., Biron K. K., Lehrman S. N., Barry D. W., Furman P. A. Plaque autoradiography assay for the detection and quantitation of thymidine kinase-deficient and thymidine kinase-altered mutants of herpes simplex virus in clinical isolates. Antimicrob Agents Chemother. 1985 Aug;28(2):181–187. doi: 10.1128/aac.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Chen M. S., Ghazzouli I., Saral R., Burns W. H. Drug resistance patterns of herpes simplex virus isolates from patients treated with acyclovir. Antimicrob Agents Chemother. 1985 Dec;28(6):740–744. doi: 10.1128/aac.28.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Corey L., Dekket C., Barry D. W. In vitro sensitivity to acyclovir in genital herpes simplex viruses from acyclovir-treated patients. J Infect Dis. 1983 Nov;148(5):868–875. doi: 10.1093/infdis/148.5.868. [DOI] [PubMed] [Google Scholar]
- McLaren C., Ellis M. N., Hunter G. A. A colorimetric assay for the measurement of the sensitivity of herpes simplex viruses to antiviral agents. Antiviral Res. 1983 Nov;3(4):223–234. doi: 10.1016/0166-3542(83)90001-3. [DOI] [PubMed] [Google Scholar]
- McLaren C., Sibrack C. D., Barry D. W. Spectrum of sensitivity of acyclovir of herpes simplex virus clinical isolates. Am J Med. 1982 Jul 20;73(1A):376–379. doi: 10.1016/0002-9343(82)90126-7. [DOI] [PubMed] [Google Scholar]
- Norris S. A., Kessler H. A., Fife K. H. Severe, progressive herpetic whitlow caused by an acyclovir-resistant virus in a patient with AIDS. J Infect Dis. 1988 Jan;157(1):209–210. doi: 10.1093/infdis/157.1.209. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Harrington J. E. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob Agents Chemother. 1982 Jul;22(1):71–77. doi: 10.1128/aac.22.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks S. L., Wanklin R. J., Reece D. E., Hicks K. A., Tyler K. L., Coen D. M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989 Dec 1;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- Safrin S., Assaykeen T., Follansbee S., Mills J. Foscarnet therapy for acyclovir-resistant mucocutaneous herpes simplex virus infection in 26 AIDS patients: preliminary data. J Infect Dis. 1990 Jun;161(6):1078–1084. doi: 10.1093/infdis/161.6.1078. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibrack C. D., McLaren C., Barry D. W. Disease and latency characteristics of clinical herpes virus isolated after acyclovir therapy. Am J Med. 1982 Jul 20;73(1A):372–375. doi: 10.1016/0002-9343(82)90125-5. [DOI] [PubMed] [Google Scholar]
- Straus S. E., Takiff H. E., Seidlin M., Bachrach S., Lininger L., DiGiovanna J. J., Western K. A., Smith H. A., Lehrman S. N., Creagh-Kirk T. Suppression of frequently recurring genital herpes. A placebo-controlled double-blind trial of oral acyclovir. N Engl J Med. 1984 Jun 14;310(24):1545–1550. doi: 10.1056/NEJM198406143102401. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Jones J. C., Ressel S. J., Fralish F. A. Thymidine plaque autoradiography of thymidine kinase-positive and thymidine kinase-negative herpesviruses. J Clin Microbiol. 1983 Jan;17(1):122–127. doi: 10.1128/jcm.17.1.122-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B. Role of herpes simplex virus thymidine kinase expression in viral pathogenesis and latency. Intervirology. 1991;32(2):76–92. doi: 10.1159/000150188. [DOI] [PubMed] [Google Scholar]
- Wade J. C., Newton B., McLaren C., Flournoy N., Keeney R. E., Meyers J. D. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double-blind trial. Ann Intern Med. 1982 Mar;96(3):265–269. doi: 10.7326/0003-4819-96-3-265. [DOI] [PubMed] [Google Scholar]



