Abstract
Tarsiers and extinct tarsier-like primates have played a central role in views of primate phylogeny and evolution for more than a century. Because of the importance of tarsiers in so many primatological problems, there has been particular interest in questions about the origin of tarsier specializations and the biogeography of early tarsioid radiations. We report on a new fossil of rare Afrotarsius that shows near identity to modern Tarsius in unique specializations of the leg, which provides information about the locomotor behavior and clarifies the phylogenetic position of this previously controversial primate. These specializations constitute evidence that Afrotarsius is a tarsiid, closely related to extant Tarsius; hence, it is now excluded from being a generalized sister taxon to Anthropoidea.
The hypothesis that extant tarsiers and anthropoids are phylogenetically linked to each other more closely than either is to extant strepsirhines has a long history in evolutionary primatology (1–4). The hypothesis rests primarily on soft tissue structures not preserved in the fossil record, and so interpretation of the phylogenetic position of many fossil tarsier-like primates has remained enigmatic. Recently, it has been proposed that a fossil tarsioid from the early Oligocene of Egypt, Afrotarsius, is the one most closely related to Anthropoidea (5–7). Afrotarsius is but one representative of the infraorder Tarsiiformes, which contains dozens of Eocene genera classified in the family Omomyidae (8), and other, more problematic tarsier-like primates known from the early Tertiary of Asia (Eosimias, Xanthorhysis, Tarsius eocaenus). Afrotarsius and the Asian taxa have been problematic because their fossil record is poor; Eosimias is known by a few jaws, including a complete lower dentition, from middle Eocene deposits of China, the same deposits that have yielded dental remains of Xanthorhysis and T. eocaenus (9, 10). Afrotarsius is known only by a single mandible with parts of five teeth from early Oligocene deposits in the Fayum, Egypt (11). Eosimias has been placed in a new family, Eosimiidae, which has been interpreted by some as an ancestral group of anthropoids (7, 9, 12). Afrotarsius was tentatively classified in the modern family Tarsiidae on the basis of one partial dentition (11). Subsequently, a new family, Afrotarsiidae, was erected for this fragmentary jaw (5), a taxon favored by those who have suggested that Afrotarsius may be the sister taxon of Anthropoidea (5–7). Another related suggestion is that Afrotarsius belongs in the same family as Eosimias (7). Most recently, the lone specimen of Afrotarsius was illustrated alongside the nearly identical comparable elements of Tarsius to show that there is no anatomical basis in the teeth for creating a new family for it or for transferring it away from Tarsiidae (13, 14).
Phylogenetic and taxonomic assessments of early Tertiary tarsioids are difficult when based only on teeth because the tarsier dentition is persistently primitive in most respects. Specializations of the post-canine teeth compelling enough to serve as a reliable foundation for phylogenetic interpretation are difficult to find and agree upon. Most of the postcranial specializations of modern tarsiers, now restricted to several islands of Southeast Asia (15), are locomotor adaptations associated with vertical clinging and leaping. These adaptations involve mainly elongation and increased mass of hindlimb elements; the hind leg is over 1.5 times the length of the trunk, and the femur and tibia are about twice the length of the humerus and radius (intermembral index of 55). The calcaneum and navicular are greatly elongated, and the fibula is reduced and fused to the tibia. The weight of the proximal hip musculature is only 0.8% of total body mass, while that of the thigh is 6.0%, the calf 2.2%, and the foot 1.5% (16, 17).
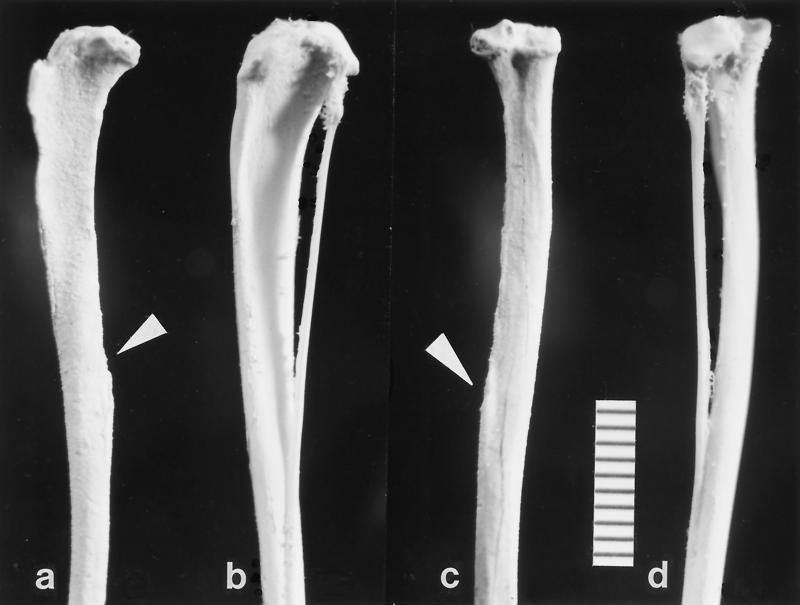
The new fossil from Egypt (Fig. 1), is a left tibiofibula lacking the distal end; it was found in 1997 on quarry M, the same quarry at which the type specimen of Afrotarsius was recovered (11). Quarry M is located in the upper sequence of the Jebel Qatrani Formation [early Oligocene (18)], at a stratigraphic level that yields the anthropoids Aegyptopithecus zeuxis, Propliopithecus chirobates, Apidium phiomense, Parapithecus grangeri, and Qatrania fleaglei. The tibiofibula is attributed to Afrotarsius on the basis of their common location on quarry M, by the unique tarsier specializations of the tibiofibula, and by size, with both the jaw and the limb bone being slightly smaller than the comparable elements in Tarsius syrichta [first molar length averages 2.8 mm in Tarsius bancanus, 2.6 mm in T. syrichta, 2.2 mm in Tarsius spectrum, and 2.0 mm in Tarsius pumilus (15), while that of the fossil is 2.45 mm (11)].
Figure 1.
Left tibiofibula (DPC 17961) of Afrotarsius chatrathi (a and c) and Tarsius syrichta (b and d) viewed laterally (a and b) and posteriorly (c and d). The fibular portion is missing in the A. chatrathi, but the point of attachment is clearly visible (arrowheads). In addition, the fossil has suffered damage on its anterior crest and the posterolateral lip of the tibial plateau. Note the posterior inclination of the tibial plateau, prominent lateral intercondylar tubercle, and the gracility and curvature of the tibial shaft. (Bar scale, 10 mm.)
In terms of overall size and shape and in most anatomical details the Oligocene tibia closely resembles that of modern Tarsius. The fossil is the proximal 45.8 mm of a left tibia (we estimate total tibial length to be approximately 55 mm). The delicate crests of the specimen are damaged, and the unfused portion of the fibula is missing. The shaft is very gracile, with the proximal end markedly compressed mediolaterally, an adaptation for resisting bending moments in the sagittal plane during leaping. The proximal shaft is also very slightly convex anteriorly but not nearly as much as in other modern primates, or in nontarsioid fossil primates. In anterior view, the proximal shaft and anterior tibial crest are bowed so that it is concave laterally (Fig. 1).
The tibial plateau is tilted backward, indicating a flexed knee position. Both medial and lateral condylar surfaces are concave, with their axis oriented anteroposteriorly, thus limiting motion at the knee to uniaxial flexion and extension. This is seen as a mechanism to maximize propulsive effectiveness in the sagittal plane. In most extant prosimians, except tarsiers, the lateral condyle has a much more convex surface than the medial one, and it extends further posteriorly (in anthropoid primates the medial condyle is often larger than the lateral). The tibia of Afrotarsius resembles that of tarsiers in that the condyles are roughly coequal in size. As in Tarsius, there is no appreciable development of a medial intercondylar tubercle, but the lateral tubercle is prominent.
The anterior border of the shaft forms a prominent crest or bony flange. The most proximal portion of the anterior tibial crest is broken away in the fossil, but on the basis of the orientation of the preserved part of the crest the attachment for the patellar tendon must have been very high and anteriorly situated as it is in modern tarsiers and galagos, an adaptation for increasing the leverage of the quadriceps femoris tendon, a major leg extensor. Like the condition in modern tarsiers, there is no well marked distal tibial tuberosity as there is in most other extant and extinct primates for insertion of the muscles gracilis and sartorius (19, 20). The more proximal insertion of these muscles in tarsiers, near the attachment for the patellar ligament, is considered a leaping adaptation. The subcutaneous medial surface of the proximal shaft is convex. The lateral surface of the proximal shaft between the anterior crest and interosseous border is a broad concavity for origin of an apparently large tibialis anterior muscle, a powerful inverter and dorsiflexor of the ankle. The gracility and curvature of the shaft, the orientation of the tibial plateau, the structure of the condyles and intercondylar tubercles, and the structures for muscle and tendon attachment, all stamp the fossil as tarsioid, even without considering the most salient feature, the fusion of tibia and fibula.
Among primates, a fused tibiofibula is found only in Tarsius and the Eocene microchoerine Necrolemur. In Tarsius, the tibiofibula is synostosed for about 60% of the tibia’s length, but in Necrolemur for only about 37% of its length (21). In the fossil specimen, the length of the unfused portion of the fibula is 20.5 mm, indicating that the two bones are co-ossified for 45% of the length of the preserved shaft, or about 60% of the estimated total length of the bone—i.e., virtually identical to the percentage in modern tarsiers. The proximal facet for the fibular articulation is broken off with the edge of the lateral tibial plateau; the distal fusion of the two bones closely resembles the condition in Tarsius, where the small fibula becomes completely incorporated in the tibia without a visible suture line or groove, unlike the case in Necrolemur, where a groove persists.
Parapithecids, like all other anthropoids, have unfused tibia and fibula. Apidium is distinctive in having the distal 40% of the tibia and fibula pressed together, and in having the proximal part of the shaft mediolaterally compressed (22, 23). This splint-like relationship of the distal fibula to the tibia also occurs in Microcebus and some platyrrhines, but not to the extent seen in Apidium. The condition in Apidium has suggested to some researchers a phylogenetic relationship to the fused tibiofibula of tarsiers, but this interpretation is difficult to sustain. For one, it would follow that other anthropoids have reverted back to the more primitive primate condition (which may be possible, but not parsimonious). More importantly, the hypothesis is illogical in light of the fusion of the tibiofibula in Necrolemur, an animal very convincingly affined with Tarsius on the basis of many cranial features functionally independent of the tibiofibula (24). The only viable way to retain parapithecids in a clade with tarsiers based on the extent of leg bone appression is to place parapithecids as the sister taxon of a combined clade containing Tarsiidae and Microchoerinae. In such a clade, the supposed anthropoid-like features of the tarsier cranium [partial “postorbital closure” and anterior position of posterior carotid foramen (25, 26)] are obviated as potential synapomorphies because they are absent from Necrolemur. On the other hand, rejecting the idea that the well-documented, detailed cranial and postcranial specializations shared by Necrolemur and Tarsius are phylogenetically meaningful begs the question of how characters for alternate hypotheses are being weighted and of why a heavily weighted character (the tibia–fibula relationship) is utilized selectively. The hypothesis that an unfused tibia and fibula in Apidium signifies a relationship to the solidly fused tibiofibula of Tarsius or Afrotarsius cannot be upheld unless a convincing post hoc explanation can dismiss Necrolemur.
When the initial jaw was found, Simons and Bown (11) tentatively assigned Afrotarsius to the modern family Tarsiidae, although they noted the possibility that it might prove to be more closely allied with the microchoerine Pseudoloris: “because of the fragmentary nature of the only known specimen of this new primate, allocation of Afrotarsius to either Omomyidae or Tarsiidae is necessarily provisional.” This taxonomically conservative and anatomically justifiable action became the subject of debate (5–7, 13), but the new tibiofibula resolves the taxonomic ambiguity; Afrotarsius can be assigned confidently to Tarsiidae on the basis of shared specializations of the tibiofibula. The detailed similarities between Afrotarsius and Tarsius in their specialized leg and primitive teeth, separated in time by 32 million years, suggest that Tarsius really is a “living fossil” (27). Of the dozen or so extant primate families, Tarsiidae has the most ancient fossil record (9–10).
By linking Afrotarsius with Tarsius, the suggestion that Afrotarsius is the sister taxon of Anthropoidea is necessarily refuted (5–7). Furthermore, Anthropoidea is unlikely to be the sister of the Tarsius–Afrotarsius clade because that position is more confidently and robustly occupied by microchoerines, or other omomyids (28). Wedging anthropoids within this combined Tarsius–Afrotarsius–Microchoerinae clade is problematic; the stronger the phylogenetic tie becomes between Tarsius and early Tertiary tarsiiforms with increasing knowledge of the fossil record, the more obviously independent of anthropoids becomes the derivation of the odd tarsier postorbital plate and bullar features (28–30). As for the suggestion that Afrotarsius and Eosimias belong in a single family, this is certainly consistent with the morphology of the dentitions, which are very similar to each other and to Tarsius. We interpret Eosimias as a tarsioid retaining the primitive primate condition of two pairs of lower incisors; the putative synapomorphies (9, 12) alleged to link Eosimias to Anthropoidea are not uniquely distributed to these two taxa and are insufficient to justify the phylogenetic hypothesis.
Acknowledgments
We thank the Egyptian Geological Survey and Mining Authority and the staff of the Cairo Geological Museum for cooperation and support in Egypt, and P. S. Chatrath for managing the field operations. We thank M. Dagosto for loaning us a cast of Necrolemur, and J. G. Fleagle and F. A. Ankel-Simons for review of the manuscript. Research has been funded by the National Science Foundation (SBR98-07770). This is Duke Primate Center publication no. 667.
References
- 1.Hubrecht A A W. Festschrift für C. Gegenbauer II. 1896. pp. 147–178. [Google Scholar]
- 2.Wortman J L. Am J Sci. 1904;17:203–214. [Google Scholar]
- 3.Pocock, R. I. (1918) Proc. Zool. Soc. London, 19–53.
- 4.Rasmussen D T. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 335–360. [Google Scholar]
- 5.Ginsburg L, Mein P. C R Acad Sci Ser II. 1987;304:1213–1215. [Google Scholar]
- 6.Kay R F, Williams B A. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 361–446. [Google Scholar]
- 7.Kay R F, Ross C, Williams B A. Science. 1997;275:797–804. doi: 10.1126/science.275.5301.797. [DOI] [PubMed] [Google Scholar]
- 8.Szalay F S. Bull Am Mus Nat Hist. 1976;156:157–450. [Google Scholar]
- 9.Beard K C, Qi T, Dawson M R, Wang B, Li C. Nature (London) 1994;368:604–609. doi: 10.1038/368604a0. [DOI] [PubMed] [Google Scholar]
- 10.Beard K C. Bull Carnegie Mus Nat Hist. 1998;34:260–277. [Google Scholar]
- 11.Simons E L, Bown T M. Nature (London) 1985;313:475–477. [Google Scholar]
- 12.Beard K C, Tong Y, Dawson M R, Wang J, Huang X. Science. 1996;272:82–85. [Google Scholar]
- 13.Simons E L. Ybk Phys Anthropol. 1995;38:199–238. [Google Scholar]
- 14.Simons, E. L. (1998) Folia Primatol. 69, Suppl. 1, 286–294.
- 15.Musser G G, Dagosto M. Am Mus Novitates. 1987;2867:1–53. [Google Scholar]
- 16.Grand T I, Lorenz R. Folia Primatol. 1968;9:161–181. doi: 10.1159/000155179. [DOI] [PubMed] [Google Scholar]
- 17.Schultz A H. Am J Phys Anthropol. 1962;20:1–24. doi: 10.1002/ajpa.1330200115. [DOI] [PubMed] [Google Scholar]
- 18.Kappelman J, Simons E L, Swisher C C. J Geol. 1992;100:647–668. [Google Scholar]
- 19.Gregory W K. Mem Am Mus Nat Hist. 1920;3:49–243. [Google Scholar]
- 20.Rose K D, Walker A C. Am J Phys Anthropol. 1985;66:73–89. doi: 10.1002/ajpa.1330660107. [DOI] [PubMed] [Google Scholar]
- 21.Dagosto M. Int J Primatol. 1985;6:45–75. [Google Scholar]
- 22.Fleagle J G, Simons E L. Nature (London) 1983;301:238–239. [Google Scholar]
- 23.Fleagle J G, Kay R F. J Human Evol. 1987;16:483–532. [Google Scholar]
- 24.Rosenberger A L. Folia Primatol. 1985;45:179–194. [Google Scholar]
- 25.Cartmill M. In: Evolutionary Biology of the New World Monkeys and Continental Drift. Ciochon R L, Chiarelli A B, editors. New York: Plenum; 1980. pp. 243–274. [Google Scholar]
- 26.Ross C. In: Anthropoid Origins. Fleagle J G, Kay R F, editors. New York: Plenum; 1994. pp. 469–548. [Google Scholar]
- 27.Schwartz J H. In: Living Fossils. Eldredge N, Stanley S M, editors. Berlin: Springer; 1984. pp. 38–49. [Google Scholar]
- 28.Simons E L, Rasmussen D T. Am J Phys Anthropol. 1989;79:1–23. doi: 10.1002/ajpa.1330790103. [DOI] [PubMed] [Google Scholar]
- 29.Conroy G C. Am J Phys Anthropol. 1980;52:443–451. doi: 10.1002/ajpa.1330530107. [DOI] [PubMed] [Google Scholar]
- 30.Beard K C, Krishtalka L, Stucky R K. Nature (London) 1991;349:64–67. doi: 10.1038/349064a0. [DOI] [PubMed] [Google Scholar]