Abstract
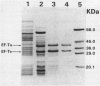
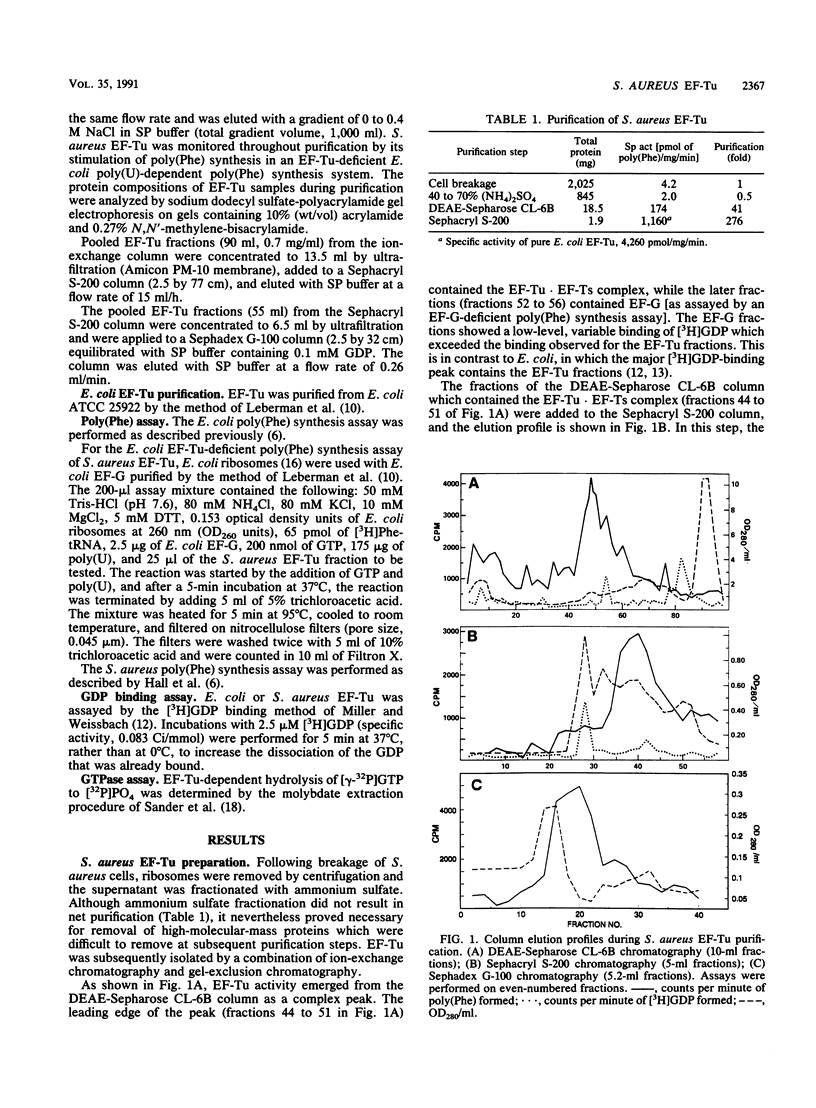
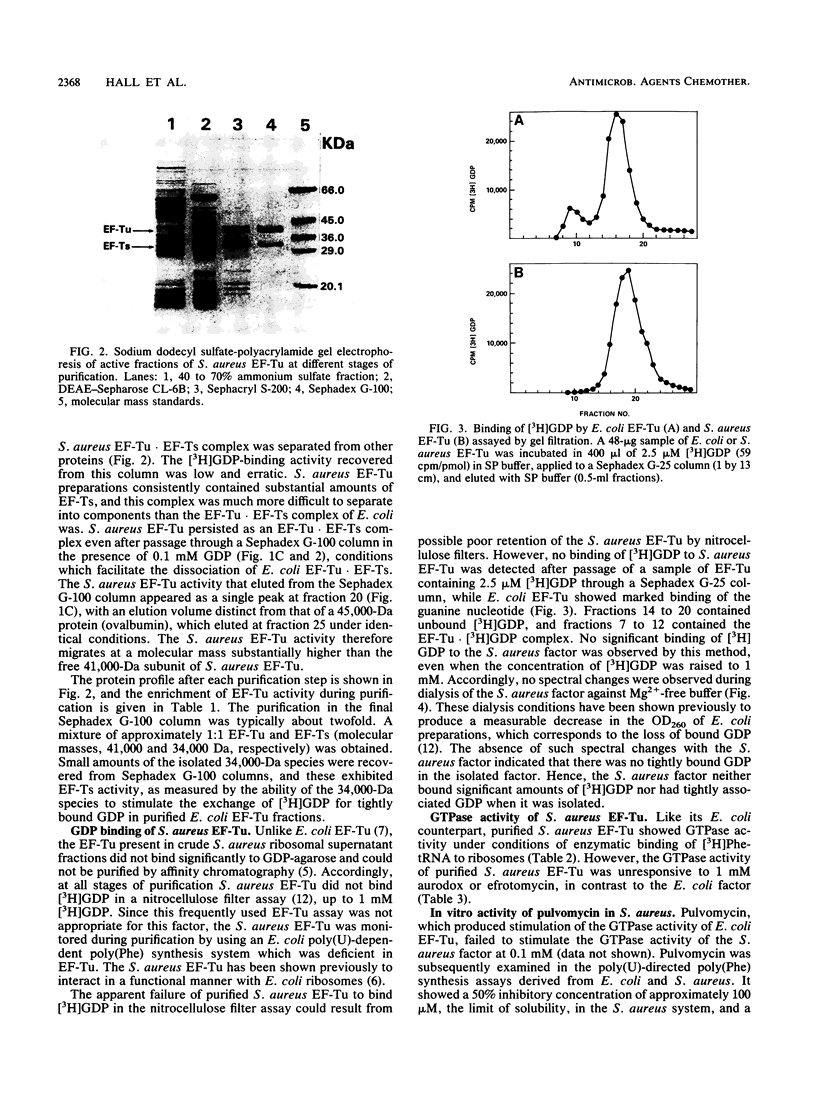
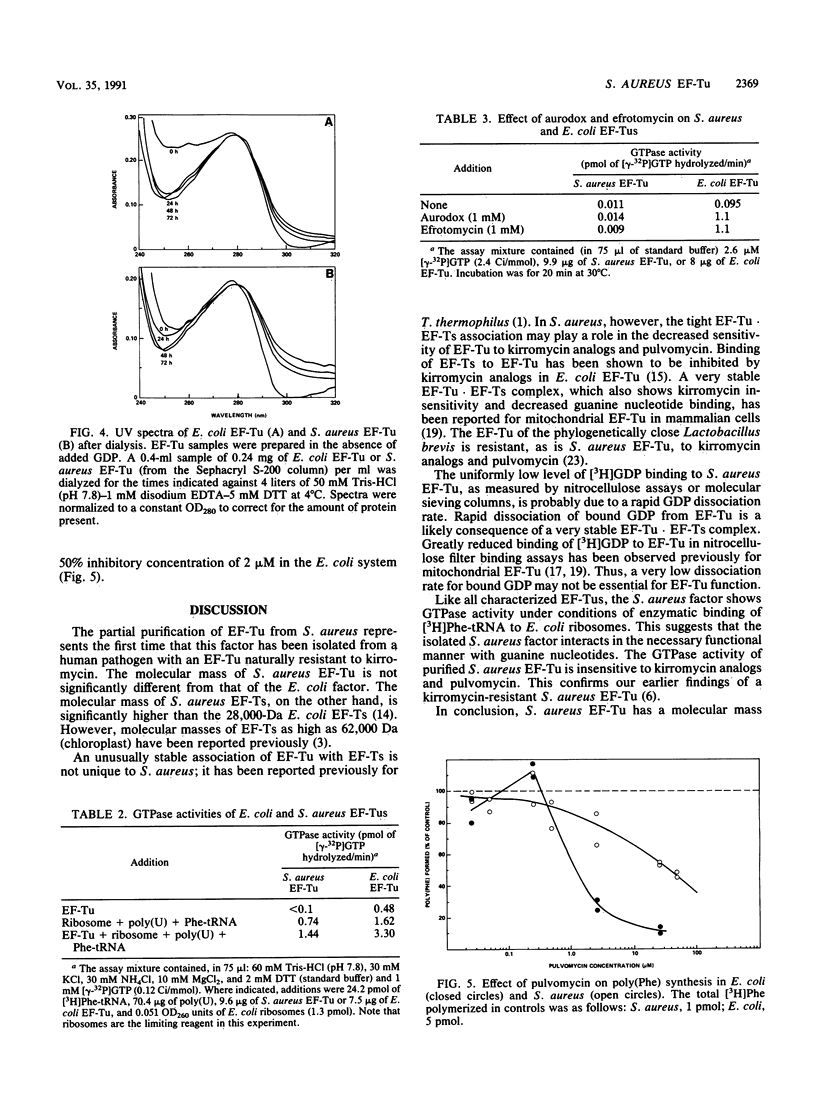
In a previous study (C. C. Hall, J. D. Watkins, and N. H. Georgopapadakou, Antimicrob. Agents Chemother. 33:322-325, 1989), the elongation factor Tu (EF-Tu) from Staphylococcus aureus was found to be insensitive to a series of kirromycin analogs which were inhibitory to the EF-Tu from Escherichia coli. In the present study, the EF-Tu from S. aureus was partially purified and characterized. Its apparent molecular mass was approximately 41,000 Da, and the enzyme copurified with EF-Ts (molecular mass, 34,000 Da). S. aureus EF-Tu differed from its E. coli counterpart in that it bound negligible amounts of [3H]GDP, in addition to being insensitive to pulvomycin and aurodox (50% inhibitory concentrations, approximately 100 and 1,000 microM, respectively, versus 2 and 0.2 microM, respectively, for E. coli). The results are consistent with the formation of a stable EF-Tu.EF-Ts complex that affects the interaction of EF-Tu with guanine nucleotides and inhibitors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Ota Y., Arai N., Nakamura S., Henneke C., Oshima T., Kaziro Y. Studies on polypeptide-chain-elongation factors from an extreme thermophile, Thermus thermophilus HB8. 1. Purification and some properties of the purified factors. Eur J Biochem. 1978 Dec;92(2):509–519. doi: 10.1111/j.1432-1033.1978.tb12773.x. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Methicillin-resistant staphylococci. Clin Microbiol Rev. 1988 Apr;1(2):173–186. doi: 10.1128/cmr.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L., Brot N., Weissbach H. Purification of Euglena gracilis chloroplast elongation factor Ts. J Biol Chem. 1981 Aug 10;256(15):7796–7799. [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. C., Watkins J. D., Georgopapadakou N. H. Effects of elfamycins on elongation factor Tu from Escherichia coli and Staphylococcus aureus. Antimicrob Agents Chemother. 1989 Mar;33(3):322–325. doi: 10.1128/aac.33.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. Affinity purification of elongation factors Tu and Ts. FEBS Lett. 1977 Jul 1;79(1):8–10. doi: 10.1016/0014-5793(77)80338-4. [DOI] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Elongation factor Tu and the aminoacyl-tRNA-EFTu-GTP complex. Methods Enzymol. 1974;30:219–232. doi: 10.1016/0076-6879(74)30024-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A., Swart G. W. Mechanism of action of kirromycin-like antibiotics. Annu Rev Microbiol. 1985;39:557–577. doi: 10.1146/annurev.mi.39.100185.003013. [DOI] [PubMed] [Google Scholar]
- Pasquale D. K. Characteristics of Medicare-eligible home care clients. Public Health Nurs. 1988 Sep;5(3):129–134. doi: 10.1111/j.1525-1446.1988.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. P., Bodley J. W. Purification and characterization of Saccharomyces cerevisiae mitochondrial elongation factor Tu. J Biol Chem. 1987 Aug 15;262(23):10955–10959. [PubMed] [Google Scholar]
- Sander G., Marsh R. C., Voigt J., Parmeggiani A. A comparative study of the 50S ribosomal subunit and several 50S subparticles in EF-T-and EF-G-dependent activities. Biochemistry. 1975 May 6;14(9):1805–1814. doi: 10.1021/bi00680a001. [DOI] [PubMed] [Google Scholar]
- Schwartzbach C. J., Spremulli L. L. Bovine mitochondrial protein synthesis elongation factors. Identification and initial characterization of an elongation factor Tu-elongation factor Ts complex. J Biol Chem. 1989 Nov 15;264(32):19125–19131. [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (second of two parts). N Engl J Med. 1984 May 31;310(22):1437–1442. doi: 10.1056/NEJM198405313102206. [DOI] [PubMed] [Google Scholar]
- Wörner W., Wolf H. Kirromycin-resistant elongation factor Tu from wild-type of Lactobacillus brevis. FEBS Lett. 1982 Sep 20;146(2):322–326. doi: 10.1016/0014-5793(82)80944-7. [DOI] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]