Abstract
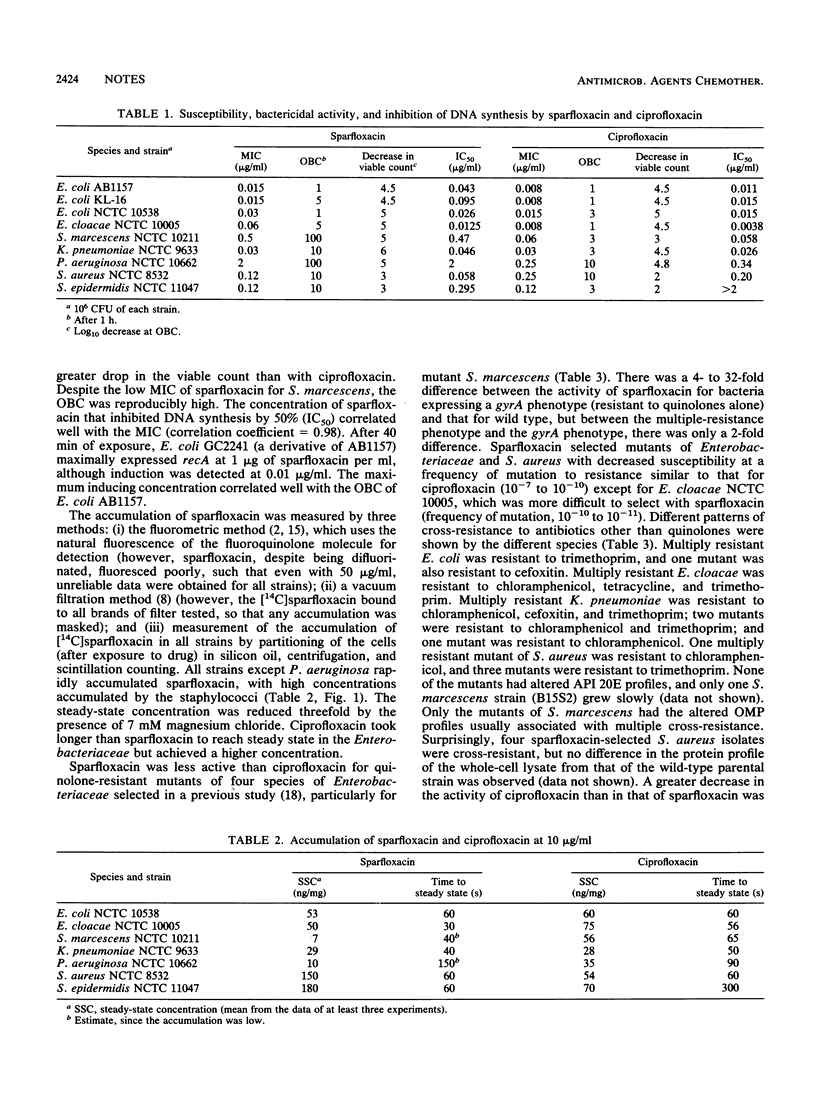
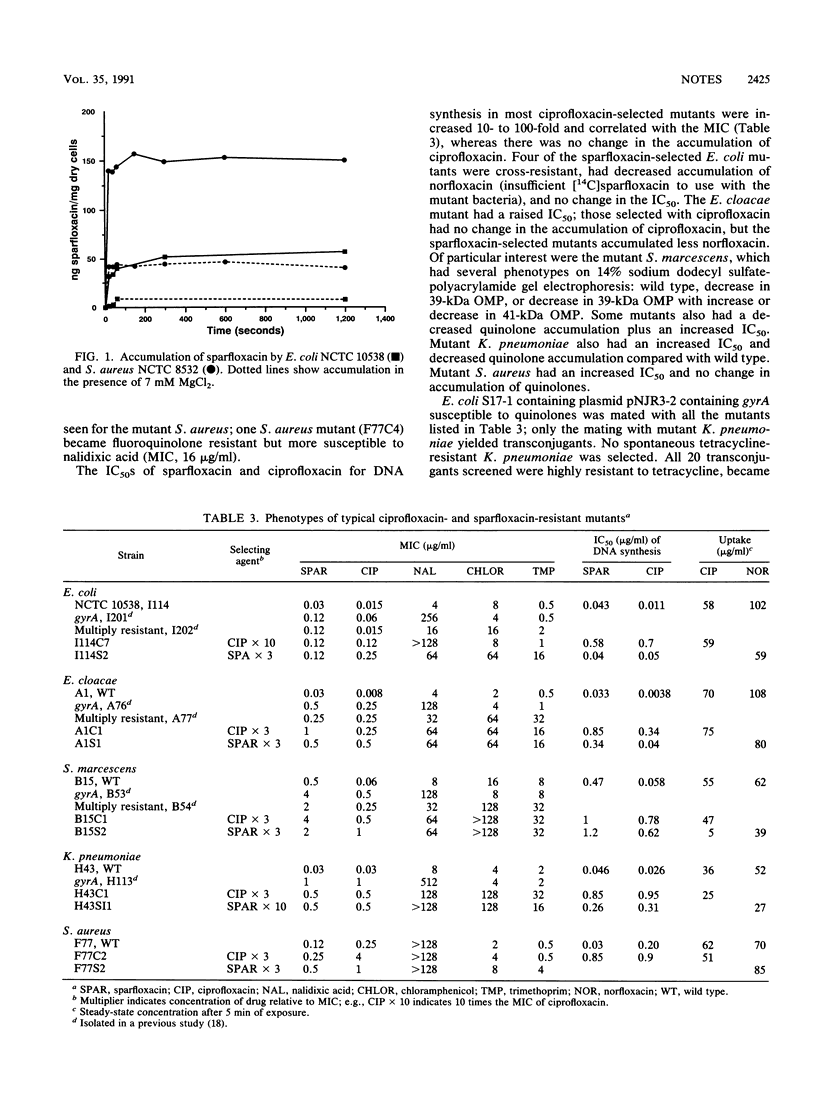
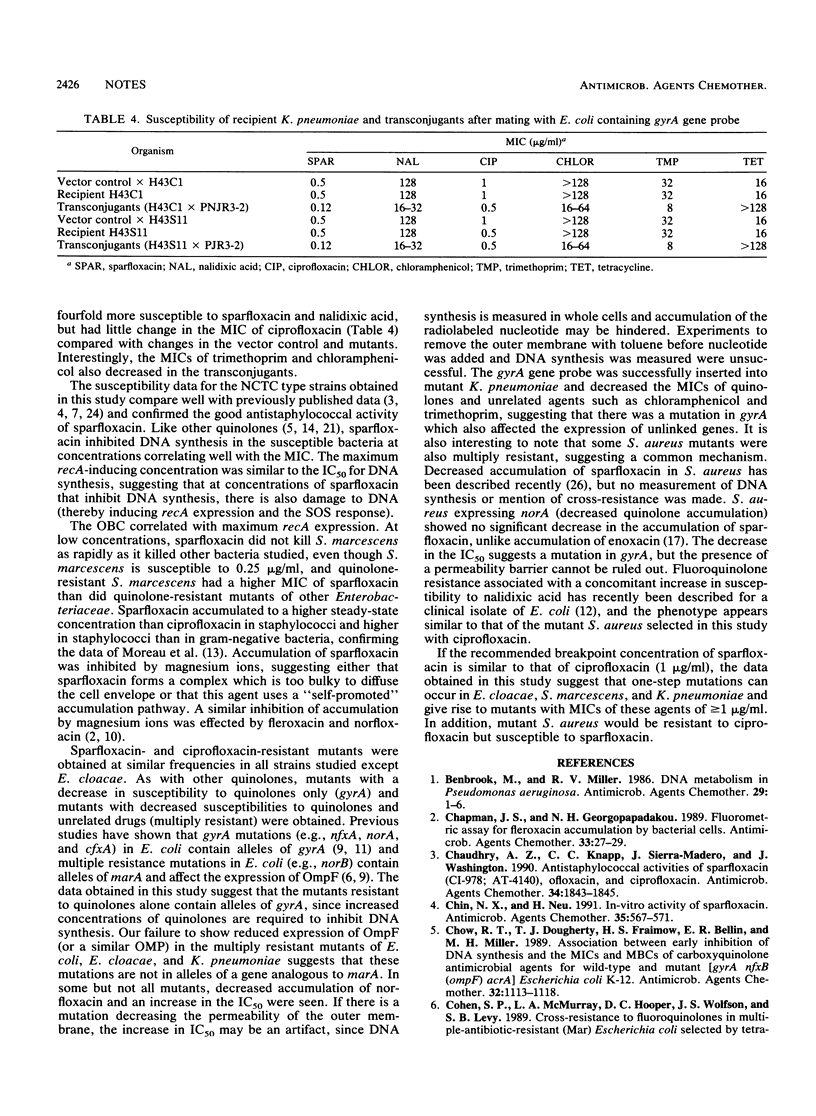
The inhibition of DNA synthesis by sparfloxacin; accumulation of sparfloxacin into members of the family Enterobacteriaceae, Pseudomonas aeruginosa, and staphylococci; induction of recA in Escherichia coli; and the optimum bactericidal concentration (OBC) were measured, and killing kinetics at the OBC were estimated. The OBC and maximum recA-inducing concentration in E. coli were both 1 microgram of sparfloxacin per ml. Accumulation was rapid; two- to threefold more sparfloxacin than ciprofloxacin accumulated in staphylococci and more sparfloxacin accumulated in staphylococci than in gram-negative bacteria. Laboratory mutants with decreased susceptibilities to quinolones alone or multiply resistant were selected from the Enterobacteriaceae and Staphylococcus aureus by using sparfloxacin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Fluorometric assay for fleroxacin uptake by bacterial cells. Antimicrob Agents Chemother. 1989 Jan;33(1):27–29. doi: 10.1128/aac.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A. Z., Knapp C. C., Sierra-Madero J., Washington J. A. Antistaphylococcal activities of sparfloxacin (CI-978; AT-4140), ofloxacin, and ciprofloxacin. Antimicrob Agents Chemother. 1990 Sep;34(9):1843–1845. doi: 10.1128/aac.34.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Gu J. W., Yu K. W., Zhang Y. X., Neu H. C. In vitro activity of sparfloxacin. Antimicrob Agents Chemother. 1991 Mar;35(3):567–571. doi: 10.1128/aac.35.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Hooper D. C., Wolfson J. S., Levy S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989 Aug;33(8):1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A., Andrews J. M., Ashby J. P., Matthews R. S., Wise R. In-vitro activity of sparfloxacin, a new quinolone antimicrobial agent. J Antimicrob Chemother. 1990 Nov;26(5):667–676. doi: 10.1093/jac/26.5.667. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Piddock L. J., Wise R. The accumulation of five quinolone antibacterial agents by Escherichia coli. J Antimicrob Chemother. 1990 Mar;25(3):319–333. doi: 10.1093/jac/25.3.319. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniot-Ville N., Guibert J., Moreau N., Acar J. F., Collatz E., Gutmann L. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob Agents Chemother. 1991 Mar;35(3):519–523. doi: 10.1128/aac.35.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau N. J., Robaux H., Baron L., Tabary X. Inhibitory effects of quinolones on pro- and eucaryotic DNA topoisomerases I and II. Antimicrob Agents Chemother. 1990 Oct;34(10):1955–1960. doi: 10.1128/aac.34.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Hall M. C., Wise R. Mechanism of action of lomefloxacin. Antimicrob Agents Chemother. 1990 Jun;34(6):1088–1093. doi: 10.1128/aac.34.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Traynor E. A., Wise R. A comparison of the mechanisms of decreased susceptibility of aztreonam-resistant and ceftazidime-resistant Enterobacteriaceae. J Antimicrob Chemother. 1990 Dec;26(6):749–762. doi: 10.1093/jac/26.6.749. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Walters R. N., Diver J. M. Correlation of quinolone MIC and inhibition of DNA, RNA, and protein synthesis and induction of the SOS response in Escherichia coli. Antimicrob Agents Chemother. 1990 Dec;34(12):2331–2336. doi: 10.1128/aac.34.12.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990 Oct;34(10):1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston K. V., Nguyen H., Messer M., LeBlanc B., Ho D. H., Bodey G. P. In vitro activity of sparfloxacin (CI-978; AT-4140) against clinical isolates from cancer patients. Antimicrob Agents Chemother. 1990 Nov;34(11):2263–2266. doi: 10.1128/aac.34.11.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubach W. H., Whitmer J. D., Davern C. I. Genetic control of DNA initiation in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):205–221. doi: 10.1016/0022-2836(73)90107-1. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Kojima T., Inoue M., Mitsuhashi S. Uptake of sparfloxacin and norfloxacin by clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):368–370. doi: 10.1128/aac.35.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]



