Abstract
Somatic-cell hybrids have been shown to maintain the correct epigenetic chromatin states to study developmental globin gene expression as well as gene expression on the active and inactive X chromosomes. This suggests the potential use of somatic-cell hybrids containing either a maternal or a paternal human chromosome as a model system to study known imprinted genes and to identify as-yet-unknown imprinted genes. Testing gene expression by using reverse transcription followed by PCR, we show that functional imprints are maintained at four previously characterized 15q11–q13 loci in hybrids containing a single human chromosome 15 and at two chromosome 11p15 loci in hybrids containing a single chromosome 11. In contrast, three γ-aminobutyric acid type A receptor subunit genes in 15q12–q13 are nonimprinted. Furthermore, we have found that differential DNA methylation imprints at the SNRPN promoter and at a CpG island in 11p15 are also maintained in somatic-cell hybrids. Somatic-cell hybrids therefore are a valid and powerful system for studying known imprinted genes as well as for rapidly identifying new imprinted genes.
Keywords: Beckwith–Weidemann syndrome/gene expression/imprinting/methylation/Prader–Willi syndrome
The process of genomic imprinting differentially marks genes in the germ line such that gene expression in the embryo and adult depends on the sex of the transmitting parent. This unequal parental contribution to offspring was first demonstrated by pronuclear transplantation studies in the mouse (1) and was further supported by breeding experiments giving rise to mice with uniparental disomy (UPD) for portions of their genome (2). Imprinting effects were shown for 10 regions on 6 different chromosomes, with phenotypes ranging from embryonic lethality to more subtle growth abnormalities (1, 2). To date, most of the imprinted genes in the mouse have been shown to have imprinted homologs in humans. Based on results from mice generated to have UPD for portions of their genome (2), one may expect 10 or more imprinted domains in humans, each containing multiple imprinted loci.
Recently, genomic imprinting has been shown to be involved in the etiology of human disease. Prader–Willi syndrome (PWS) and Angelman syndrome (AS) are clinically distinct disorders caused by the lack of expression of genes in chromosome 15q11–q13 (3). Most commonly, PWS arises from a paternally inherited de novo 4-Mb deletion of this region (≈75% of cases) or from a maternally inherited UPD (≈25% of cases). Conversely, AS most frequently results from the maternal inheritance of an identical deletion, and paternal UPD has been shown in approximately 2% of cases. Some AS and PWS patients have mutations in the imprinting process (3, 4). Most recently, about 5% of AS patients have been shown to have mutations in the UBE3A gene (5), although the basis for AS in the remaining 10–15% of patients is unclear. Similarly, Beckwith–Weidemann syndrome, a fetal overgrowth syndrome associated with a high incidence of Wilms tumors, is caused both by maternal chromosome 11p15 loss or rearrangement and paternal isodisomy (6). Furthermore, mutations in the imprinted p57KIP2 (CDKN1C) gene have been discovered in some patients with Beckwith–Weidemann syndrome (7), and the maternal (expressed) copy of this gene has been shown to be preferentially deleted in many lung cancers (8) and down-regulated in Wilms tumors (9, 10). Although the aforementioned phenotypes are readily discernible, it is likely that many more genes are subject to genomic imprinting, defects in which may lead to more subtle phenotypes.
With the importance of genomic imprinting in human disease and the relative frequency with which genes in imprinted domains are being discovered in both the mouse and human, there is a critical need for a general model system with which to identify novel imprinted genes in humans and to further characterize and potentially manipulate known imprinted genes. Currently, cell lines from patients with UPD or deletions of specific parental origins are available for very few chromosomes (11). In contrast, the availability of interspecific backcrosses makes determination of allele specificity of expression relatively easy in the mouse (12–14). However, because not all genes may show conserved imprinting, e.g., IGF2R (15), it is important to test each candidate human gene.
Previously, it has been demonstrated (16) that somatic-cell hybrids produced by fusing human erythroid cells expressing an embryonic, fetal, or adult β-like globin gene with mouse erythroleukemia cells, an adult cell type, retain expression of the appropriate human globin gene, indicating that the chromosomal state of the β-globin locus is transferred intact in these hybrid cells. Somatic-cell hybrids also have been shown to faithfully maintain the active or inactive state of the X chromosome and thus have been instrumental in determining which genes are subject to and which genes escape from X inactivation (17). Combined, these data suggest that the epigenetic state of a chromosome can be stably transferred into cells of a different developmental and/or differentiation stage and maintain its initial pattern of gene expression. Based on these results, and to provide a general model system for assaying novel imprinted genes, we have tested somatic-cell hybrids containing individual human chromosomes for the maintenance of characteristics of imprinted genes, including monoallelic expression and differential DNA methylation of maternal and paternal alleles. Here we show that imprinted gene expression and DNA methylation of critical CpG islands are faithfully maintained in somatic-cell hybrids. We also demonstrate that the γ-aminobutyric acid type A (GABAA) receptor genes in 15q12–q13 are not imprinted, contrary to a recent report (18).
MATERIALS AND METHODS
Cell Culture and Hybrids.
Standard tissue-culture techniques were used to propagate the hybrid cell lines (Tables 1 and 2) used in this study. Chromosome 11 hybrids (Table 2) were obtained from the NIGMS Coriell Cell Repositories (Camden, NJ). It is important to note that hybrid cell lines may be karyotypically unstable and should be regularly assessed for chromosome content by either cytogenetic or molecular methods. Drug selection used in tissue culture may help to stabilize the genotype of cell lines; in particular, the chromosomes 15 in hybrids A15 and A9+15 confer neomycin resistance on those hybrids (19, 20).
Table 1.
Somatic-cell hybrids used for analysis of chromosome 15
| Hybrid | Rodent background | Origin of human donor cell | Chromosomes present | Chromosome 15 imprint | Source (reference) |
|---|---|---|---|---|---|
| A15 | Mouse A9 | GM01604 Fibroblast | 15 | pat | R. Schultz (19) |
| A9 + 15 | Mouse A9 | fetal lung Fibroblast | 15 | pat | NIGMS (20) |
| A59-3az | Mouse A9 | Fibroblast | 1, 4, 8, 10, 13, 15, 22 | pat | H. F. Willard |
| t60-14 | Mouse tsA-1S9 | Fibroblast | 4, 8, 11, 13, 14, 15, 18, 21, 22, X | pat | H. F. Willard (54) |
| 20L-28 | LM/TK | GM00291A Fibroblast | t(17;1), 3, 4, 5, 6, 7, 8, 11, 13, 14, 15, 16, 20, 21 | mat | T.B.S. |
| ALA-8 | Mouse A9 | Fibroblast | 1, 2, 3, 4, 5, 6, 7, 8, t(X;9), 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, X | mat | T.B.S. (55) |
| GAR-1 | RAG | GM00806 Fibroblast | 3, 5, 8, 10, 12, 13, 15, 16, 20, X | mat | T.B.S. |
| t75-2maz-34-4a | Mouse tsA-1S9 | Fibroblast | X, 3, 6, 7, 12, 14, 15, 16, 17, 19, 20, 21 | mat | H. F. Willard |
| 15A | CHO-K1 | AML Lymphocyte | 15 + 2-3 non-random human | mat (+ pat frag. in ∼10% of cells) | NIGMS |
| 55R-16 | RAG | GM05519 Fibroblast | 1, 2, 3, t(4;11), 5, 6, 7, 8, 10, 12, 13, 15, 16, 19, 21, 22, X | mat/pat | T.B.S. (56) |
| DUA-1A | Mouse 1R | skin Fibroblast | t(X;15) (p11;q11) | pat | T.B.S. (28) |
| 2-3-4 | Mouse A9 | skin Fibroblast | t(15;19) (q12;q13.41) | pat | R.D.N. |
| t86-B1-maz1b-3a | Mouse tsA-1S9 | Fibroblast | X, 15 | pat | H. F. Willard (57) |
AML, Acute myelogenous leukemia; CHO, Chinese hamster ovary; frag., fragment; mat, maternal; pat, paternal; NIGMS, National Institute of General Medical Sciences.
Table 2.
Somatic-cell hybrids used for analysis of chromosome 11
| Somatic-cell hybrid | Rodent background | Origin of human donor cell | Chromosomes present | Chromosome 11 methylation imprint |
|---|---|---|---|---|
| GM10482A | Mouse A9 | Fibroblast | der (11) t (X;11) (q26;q23),7 | pat |
| GM10927B | CHO-K1 | Amniotic fibroblast | 11 | pat |
| GM11087A | Mouse 3T3 | Foreskin fibroblast | 11 | pat |
| GM07300 | CHL | N/A | 6,8,11,X | mat |
| GM11944 | CHO-K1 | Amniotic fibroblast | 11pter>cen. translocated to a CH chromosome | pat |
| GM11941 | Mouse L-1R | Lymphocyte | 11, Xp translocated to a mouse chromosome | pat |
| GM13400 | CHO a3 | Ewing sarcoma | der 11t(11;22)(q24;q12) | mat |
| GM11937 | CHO a3 | Lymphocyte | der(11)t(4;11)(q21;q23) | pat |
Abbreviations: pat, paternal; mat, maternal; cen, centromere; CHO, Chinese hamster ovary; CHL, Chinese hamster lung.
DNA Methylation Analysis.
DNA extraction and Southern hybridizations were performed by using standard procedures (21). For SNRPN, genomic DNA from cell lines was digested with XbaI and the methyl-sensitive NotI restriction enzymes, electrophoresed on a 0.8% gel, analyzed by Southern blotting, and hybridized with a SNRPN exon 1 probe (21). For D15S63 and ZNF127, DNA was digested with BglII and HhaI or EcoRI and HpaII and probed with PW71B or a 1.3-kb TaqI/EcoRI fragment of DN34, respectively. For the chromosome 11p15 region, genomic DNA was digested with BamHI and NotI, blotted as above, and hybridized with a probe for a differentially methylated CpG island that maps to an intron of KVLQT1 (KCNA9). The image was exposed by using a PhosphorImager (Molecular Dynamics).
RNA Extraction and Reverse Transcription–PCR (RT-PCR).
Total RNA was extracted from fibroblast cell lines twice (to remove DNA contamination) by using RNAzolB (Cinna/Biotecx Laboratories, Friendswood, TX), and 5 μg was reverse-transcribed by using SuperScriptII (GIBCO/BRL) with random hexamers as a primer, and 1/25 of the RT reaction was used for subsequent 50-μl PCR amplifications. Primers and conditions for PCR were as described: 60A and 60B for IPW (22); RN85 and RN133 for SNRPN (21); PAR5 (23); P1 and P2 followed by P3 and P4 (nested) for H19 (24). For IGF2, primers used were RN245, 5′-CTCGTGCTGCATTGCTGC-3′ and RN246, 5′-GGACTGCTTCCAGGTGTC-3′. The following conditions were used: denaturation for 30 sec at 94°C, annealing for 30 sec at 58°C, and extension for 1 min at 72°C for 35 cycles. For RPS12, primers were F, 5′-ATTCAGCTTCACCCGTAACC-3′and R, 5′-CAACCACTTTACGGGGATTC-3′. The following conditions were used: 30 sec at 94°C, 30 sec at 56°C, and 30 sec at 72°C for 35 cycles. For WT1, primers were W1, 5′-ATCCTCTGCGGAGCCCAATA-3′ and W3, 5′-ACTGTGCTGCCTGGGACA-3′, and the following conditions were used: 30 sec at 94°C, 30 sec at 55°C, and 30 sec at 72°C. For NDN (NECDIN), primers were RN700, 5′-AGCCCCAAAAGAACTCGTATT-3′ and RN709, 5′-CAGAAGGCGCACGAGCTC-3′, and the following conditions were used: 30 sec at 94°C, 30 sec at 60°C, 30 sec at 72°C. GABRA5 primers were RN786, 5′-GAGAACATCAGCACCAGCACAG-3′ and RN787, 5′-AAGACGAAGGCATAGCACACAG-3′; GABRB3 primers were RN788, 5′-AGAATCACCACGACAGCAGCAT-3′ and RN789, 5′-CCAGAAGGACACCCACGACAGA-3′; GABRG3 primers were RN790, 5′-TCACCATTCAGACATACATTCC-3′ and RN791, 5′-CATCCAGACACTCATCGCCACA-3′. Cycling conditions for the GABAA receptor genes were 35 cycles of 95°C for 30 sec, 62°C for 30 sec, and 72°C for 30 sec. Primers for TAPA1 (CD81) were TAPA1a, 5′-ACTGACTGCTTTGACCACC-3′ and TAPA1b, 5′-TCCACTCATACACGCCACC-3′ and cycling conditions as for IGF2, above. GABRA5 and GABRB3 were expressed at sufficiently high levels such that RT-PCR products could be seen directly on ethidium bromide-stained agarose gels, whereas RT-PCR products for GABRG3 had to be blotted and hybridized with a cDNA probe.
Microsatellite Marker Analysis and Expressed Polymorphisms.
Dinucleotide repeat alleles of D15S123 (Genome DataBase, Baltimore) were amplified by using 50 ng of DNA and a [γ-32P]dATP-end-labeled forward primer. PCR was carried out in a 15-μl reaction volume that included 200 mM each dATP, dCTP, dGTP, and dTTP and 1.5 μl of 10× reaction buffer, reverse primer, and 0.5 units of Taq polymerase (Boehringer Mannheim). Samples were amplified in a thermocycler for 30 cycles (95°C, 30 sec; 55°C, 30 sec; 72°C, 30 sec) followed by a 6-min 72°C incubation. Before loading, 1.5 vol of 95% formamide loading buffer was aliquoted to each sample, which was electrophoresed on a 6% polyacrylamide gel (National Diagnostics). The gel was then transferred to 3M Whatman paper, dried, and exposed to autoradiographic film (Biomax) for 16 hr. Expressed polymorphisms in IPW (22) and SNRPN (25) were analyzed as described.
RESULTS
Strategy for a Somatic-Cell Hybrid Model to Study Genomic Imprinting.
Genomic imprinting results in predominant-gene expression from one parental allele in somatic cells, although this may be subject to temporal and/or tissue-specific regulation. To test the fidelity of genomic imprints in somatic-cell hybrids, cell lines were grown to near confluency and DNA and total RNA were isolated. These lines were assayed for gene expression by using RT-PCR and for DNA methylation by using Southern blot analysis. In all cases, PCR primer pairs are specific for human sequence and are unable to amplify the orthologous rodent gene. Thirteen hybrids believed to contain a single human chromosome 15 were used for analysis of genes in the PWS/AS-critical region (Fig. 1a), and eight hybrids containing a single human chromosome 11 were used to assay genes in 11p15 (Fig. 1b). It is worth noting that our hybrid cell lines were generated by using various rodent backgrounds in the fusion process and that some hybrids contain a single human chromosome, whereas others contain a large complement of human chromosomes (Tables 1 and 2). However, neither of these variables appears to have had any effect on our assays.
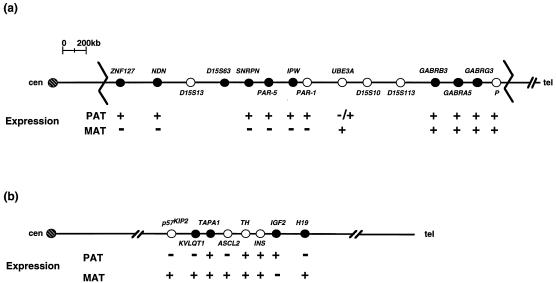
Figure 1.
Imprinted-gene maps of chromosomes 15q11–q13 (a) and 11p15 (b). The gene positions and loci assayed in somatic-cell hybrids (•) are shown. Jagged lines in a represent the common deletion breakpoints in PWS and AS patients. Symbols are: +, gene expression; −, lack of gene expression. PAT, paternal; MAT, maternal; cen, centromere; tel, telomere. The −/+ in a indicates that UBE3A is not expressed from the paternal allele in certain regions of the brain. Figure adapted from refs. 3 and 6.
Expression and Methylation Imprints in Chromosome 15q11–q13.
We first tested hybrids containing chromosome 15 for maintenance of methylation and expression imprints at the SNRPN locus. SNRPN encodes the small nuclear ribonuclear protein N, a polypeptide believed to be involved in tissue-specific splicing of mRNAs. Previous studies (21, 23, 26) have established that the SNRPN transcript is expressed only from the paternal allele and that this allele is unmethylated at the SNRPN promoter. Eleven of 13 hybrids showed only a single methylated or unmethylated allele (Fig. 2a; Table 1), and seven of these expressed the SNRPN transcript (Fig. 3a; Table 1). Importantly, expression and methylation patterns were concordant, so that hybrids that were unmethylated at the SNRPN promoter expressed the transcript, whereas those that were methylated did not express the transcript. All hybrids expressed the nonimprinted chromosome 15 control gene RPS12 (Fig. 3d).
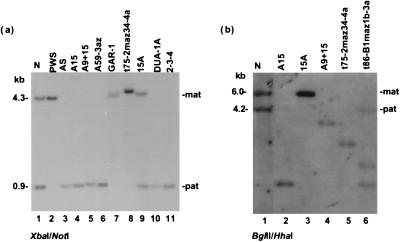
Figure 2.
DNA methylation analysis in chromosome-15 hybrids. (a) Maintenance of methylation imprints at SNRPN in somatic-cell hybrids. Normal individuals (lane 1) have both a methylated (4.3-kb) allele corresponding to the maternally inherited chromosome and an unmethylated (0.9-kb) allele corresponding to the paternally inherited chromosome. PWS deletion patients (lane 2) lack a paternal contribution, whereas AS deletion patients (lane 3) lack a maternal contribution. Most hybrids show either a completely methylated band, indicating the presence of only a maternal chromosome, or a completely unmethylated band, indicating the presence of only a paternal chromosome. Hybrids with both a methylated and an unmethylated band [15A and 55R-16 (latter data not shown)] were shown to contain both a maternal (mat) and a paternal (pat) human chromosome 15 (see Fig. 4a and Results). Lane 1, normal human lymphoblast; lane 2, lymphoblast from a PWS deletion patient; lane 3, lymphoblast from an AS deletion patient; lanes 4–11 contain DNAs from a subset of the chromosome-15 hybrid panel. (b) Methylation patterns are not maintained at D15S63, with most hybrids being hypomethylated. Hybrid 15A is hypermethylated, despite containing a proportion of cells with a paternally derived chromosome 15. Lane 1, normal human lymphoblast; lanes 2–6 contain DNAs from a subset of the chromosome-15 hybrid panel.
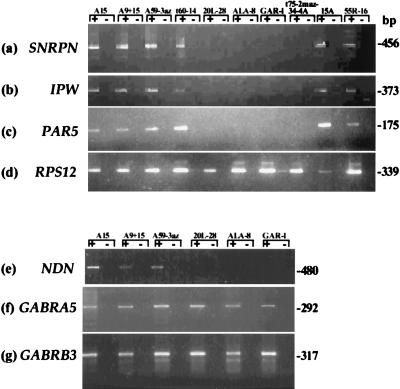
Figure 3.
Gene expression in hybrids containing human chromosome 15. Expression of SNRPN (a), IPW (b), PAR5 (c), and NDN (e) as determined by RT-PCR correlates with the SNRPN methylation data. Only those hybrids that are unmethylated at the SNRPN promoter express each of these four transcripts. The GABRA5 (f) and GABRB3 (g) receptor subunit genes are expressed in hybrids containing either a maternal or a paternal chromosome 15. For GABRG3 (data not shown), hybrid A9+15 (containing a paternal chromosome 15) and hybrid 20L-28 (containing a maternal chromosome 15) consistently showed high levels of expression. Additionally, one paternal (A59–3az2 maz) and two maternal (ALA-8, GAR-1) hybrids showed low levels of expression, whereas expression in A15 (paternal) was not detected in multiple experiments. The control gene RPS12 (d) is expressed in all hybrids. PCR was performed with (+) or without (−) reverse transcriptase.
In two hybrids, 15A and 55R-16, both a methylated and an unmethylated allele were detected (Fig. 2a; data not shown), and both hybrids express the SNRPN transcript (Fig. 3a), suggesting either a relaxation of the imprint in tissue culture or the presence of both a maternal and a paternal chromosome in these cell lines. To distinguish between these possibilities, we PCR-amplified across a previously described HphI polymorphism in exon 3 of the IPW gene (ref. 22; see Fig. 1) and digested the products to show the presence of two alleles in the DNA of each hybrid, only one of which is present in the cDNA from each hybrid (Fig. 4a; data not shown). Furthermore, fluorescence in situ hybridization using a commercial SNRPN cosmid and cells from hybrid 15A confirmed the presence of an intact chromosome 15 in most cells and a second human fragment containing SNRPN in about 10% of cells (National Institute of General Medical Sciences catalog, 1995; J. M. Amos-Landgraf and R.D.N., unpublished data). These data prove the presence of two chromosomes 15 in hybrids 15A and 55R-16. Therefore, monoallelic gene expression has been maintained in the entire panel of chromosome-15 hybrids, some of which are more than 10 yr old and have endured more than 100 passages in tissue culture.
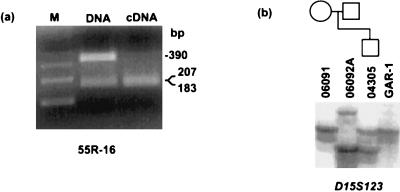
Figure 4.
Polymorphism studies of chromosome-15 hybrids. (a) Monoallelic imprinted gene expression in hybrid 55R-16 with biallelic SNRPN DNA methylation. Primers amplify an HphI polymorphism in the IPW gene in exon 3. Digestion of amplified genomic DNA (lane 2) reveals the presence of two alleles in hybrid 55R-16, only one of which is expressed (lane 3). Lane 1 contains the 123-bp ladder size marker. (b) Microsatellite analysis of hybrid cell line GAR-1 and lineage. GAR-1 contains a maternal chromosome 15 from cell line GM04305, in accordance with complete methylation at the SNRPN promoter (see Fig. 2a).
Designating chromosomes in the hybrids as either maternal or paternal was initially done on the basis of previously established imprinting patterns; however, it was a formal possibility that during the process of fusing cell lines, or after many passages in tissue culture, genomic imprints might have been reversed. To address this possibility, an informative microsatellite marker was typed in the hybrid cell line GAR-1 as well as in the hybrid cell line donor and the parents of the hybrid cell line donor (Fig. 4b). The haplotype analysis shows that the chromosome 15 contained in the GAR-1 hybrid is of maternal origin, corroborating the methylation and expression data. A second hybrid cell line containing a t(15:19) translocation previously determined to be of paternal origin (27) likewise was shown to maintain the correct paternal methylation pattern (Fig. 2a; Table 1). Therefore, it is likely that all of the hybrids that are unmethylated and express SNRPN contain a single human chromosome 15 of paternal origin, whereas all of the hybrids that are methylated at SNRPN and do not express the transcript contain a single human chromosome 15 of maternal origin.
Additionally, as methylation at the SNRPN CpG island is strictly maintained in all tested somatic-cell hybrids, we were able to use this assay to assign a parental origin to a de novo translocation chromosome in one cell line. Thus, the t(X;15) chromosome in hybrid DUA-1a (28), which is unmethylated at the SNRPN promoter, is likely of paternal origin (Fig. 2a).
To further characterize the panel of hybrids, RT-PCR was performed for the imprinted, paternally expressed IPW gene (22), the PAR5 expressed sequence tag (23), and the recently identified NDN gene (29, 30). In each hybrid, expression of IPW, PAR5, and NDN (Fig. 3) correlated with the assigned paternal or maternal origin of each chromosome 15, as inferred from the SNRPN expression and methylation data (Table 1), confirming our finding that imprinted gene expression is strictly maintained in these somatic-cell hybrids. However, methylation of D15S63, a locus previously shown to be unmethylated on the paternal allele and methylated on the maternal allele (31), was not maintained in the hybrids, with most hybrids being hypomethylated (Fig. 2b). Similarly, DNA methylation was not maintained at the ZNF127 CpG island (data not shown).
Nonimprinted Genes in Chromosome 15q11–q13.
Recently, it was reported that the human GABAA receptor genes in 15q12–q13 were imprinted and expressed exclusively from the paternal chromosome in hybrid cell lines generated by using microcell-mediated chromosome transfer (18). Because studies in the mouse suggested that these genes are not imprinted (32–34), we investigated the imprinted status of the human GABAA receptor genes in our panel of hybrids by using RT-PCR. All three genes were amplified equally well for hybrids containing either a maternal or a paternal chromosome 15 (Fig. 3 f and g; data not shown). Thus, in our system, the GABAA receptor genes are not imprinted.
Expression and Methylation Imprints in 11p15.
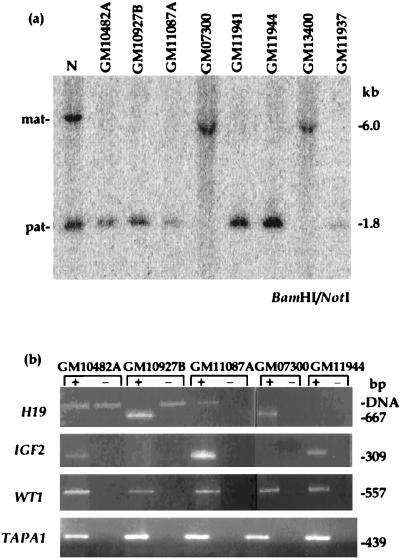
Maintenance of a methylation imprint in chromosome 11p15 was assayed at a CpG island in an intron of the KVLQT1 gene previously shown to be unmethylated on the paternal chromosome and methylated on the maternal chromosome (M.J.H., unpublished data). In each hybrid containing an individual human chromosome 11, a single unmethylated allele or methylated allele was present, consistent with retention of methylation imprints at this locus (Fig. 5a; Table 2).
Figure 5.
Methylation and gene expression analyses of hybrids containing a single human chromosome 11. (a) DNA methylation imprints are monoallelic at an intronic KVLQT1 intronic NotI site. Normal individuals have both a methylated (6.0-kb) allele corresponding to the maternally inherited chromosome (mat) and an unmethylated allele (1.8-kb) corresponding to the paternally inherited chromosome (pat). Each hybrid shows either the presence of a methylated or an unmethylated chromosome, suggesting that methylation imprints at this site are maintained in somatic-cell hybrids. (b) Each hybrid expressed either the maternal-only H19 or paternal-only IGF2 gene but never both, consistent with maintenance of functional imprints in chromosome 11p15. The primers used for H19 RT-PCR span a small intron, giving a smaller-sized product in cDNA (667 bp) compared with amplification from genomic DNA (24). Although TAPA-1 maps within the imprinted domain in 11p15, it is expressed from both maternal and paternal chromosomes, consistent with knockout studies in the mouse. The control gene, WT1, is expressed in all hybrid cell lines. + and − are as for Fig. 3.
Further studies were performed to characterize the reciprocally imprinted IGF2 and H19 genes in the hybrid cell lines carrying a single human chromosome 11. H19 is primarily expressed from the maternal allele only (24), whereas IGF2 is expressed only from the paternal allele (35). Though neither gene was highly expressed, RT-PCR data revealed that IGF2 was expressed in four hybrids and H19 was expressed in the remaining two hybrids derived from human fibroblasts (Fig. 5b; Table 2). Hybrids derived from human lymphoblasts did not express reproducibly detectable levels of IGF2 or H19 mRNA. For five of the six hybrids, the H19/IGF2 expression and KVLQT1 intronic methylation data were concordant; however, hybrid GM10927B was unmethylated at the 11p15 NotI site but expressed H19. In repeated RT-PCR experiments, weak IGF2 expression also was occasionally seen in this hybrid (data not shown). Because hybrid GM10927B was derived from amniotic fibroblasts and imprinting of H19 in the placenta has been shown to depend on developmental stage and cell type (36), fusion of a cell with biparental H19 expression could result in expression from a paternally derived chromosome. Alternatively, in this cell, H19 may be regulated independently of IGF2 (37) or the domain containing the KVLQT1 intronic NotI site; the latter is suggested by mouse studies (38). Expression of KVLQT1 was not detectable in any of the chromosome 11 hybrids. The control gene, WT1 (39), demonstrates polymorphic imprinting in preterm placentae and fetal brain (40) but generally shows biallelic expression, consistent with its expression in all somatic-cell hybrid lines. Also, TAPA1, a gene mapping between the imprinted KVLQT1 and ASCL2 genes (41) and which can be inferred from knockout experiments in the mouse not to be imprinted (42) was expressed from both chromosomes in our hybrid panel (Fig. 5b).
DISCUSSION
With the increased interest in the phenomenon of genomic imprinting and the rapid discovery of new transcripts in imprinted domains, there is a great need for a model system to study imprinted genes. Current methods for proving the imprinted status of a gene rely on finding a sequence polymorphism as well as informative families and can be very time consuming. Our approach for overcoming these obstacles is to assay for monoallelic gene expression from somatic-cell hybrids containing individual human chromosomes. By using this powerful system, it is possible to test for expression from one parental chromosome simply by performing RT-PCR on a panel of well-characterized hybrids. We have demonstrated that somatic-cell hybrids do maintain functional (expression) imprints with high fidelity. This has been shown for four known imprinted genes in chromosome 15q11–q13, as well as for two oppositely imprinted genes in chromosome 11p15. The single chromosome-11 hybrid that demonstrated potentially discordant results may be readily explained by the cell type used in generating this hybrid (36, 37). Indeed, this is further testament to the evidence that the transcriptional state of the chromosome at the time of fusion is retained in somatic-cell hybrids. Combined, these data suggest that somatic-cell hybrids can be used as a powerful reagent to assess whether any human gene is imprinted and to describe from which parental allele it is expressed.
By using a panel of chromosome-15 hybrids, we were able to independently demonstrate that the NDN gene is imprinted and expressed only from the paternal chromosome, as recently reported by others (29, 30). This is of particular interest with respect to the sensitivity of this method, as mouse Ndn has been shown to be expressed only in neurons by Northern blot analysis and is undetectable in other tissues by these measures (43), yet is easily detectable by RT-PCR in somatic-cell hybrids containing a paternal human chromosome 15.
Our data also refute a recent report that the three GABAA receptor subunit genes in chromosome 15q12–q13 are imprinted with exclusive expression from the paternal allele only (18). In an earlier study (44), GABRB3 was suggested to show exclusive maternally derived expression based on differential expression between hydatidiform moles (paternal genome only) and ovarian teratomas (maternal genome only); however, these tumors represent highly differentiated tissues that are not true models for genomic imprinting studies (21, 45, 46). In the mouse, all three GABAA receptor subunit genes, Gabrb3, Gabra5, and Gabrg3, show equal levels of expression in brain and other tissues after paternal or maternal inheritance of deletions spanning these genes, suggesting that none of these genes is imprinted (32, 33). Regional specific imprinting is unlikely, at least for Gabrb3, as ≈90–95% of homozygous deleted mice die as neonates, most with an associated cleft-palate phenotype (34, 47). Heterozygous Gabrb3 knockout mice also show intermediate values for mRNA levels as well as electrophysiological and electroencephalogram recording abnormalities compared with wild-type and Gabrb3-null mice (34). Although there may be cases of imprinted mouse genes in which the human gene appears not to be imprinted (e.g., IGF2R), studies on the mouse homologs of human 15q11–q13 genes have shown that all are conserved in relative chromosome position, structure, sequence, and imprinting status (3). Therefore, it is likely that the human GABAA receptor genes are nonimprinted, as shown here.
Of interest, we have found that somatic-cell hybrids are often, but not always, a reliable resource for assaying DNA methylation imprints. We were able to demonstrate faithful retention of a methylation imprint in all hybrids tested only at the SNRPN and NDN (T.G.G., J.M.G., and R.D.N., unpublished data) promoters in 15q11–q13 and at a KVLQT1 intronic CpG island in 11p15. The strict retention of the SNRPN methylation imprint could be indicative of its central importance in imprinting in this region. SNRPN is located in the middle of the 15q11–q13 imprinted domain and has been proposed to be an important component of the imprinting center involved in germ-line switching of the imprint for all imprinted genes in 15q11–q13 (3, 4). The SNRPN promoter methylation imprint, assayed here, is maintained in all somatic tissues tested to date (21). In contrast, differential methylation at ZNF127 is maintained only in the brain, with leukocytes and fibroblasts showing partial methylation on both alleles (M. T. C. Jung, C. C. Glenn, D. J. Driscoll, R.D.N., unpublished data). Thus, individual cells fused in generating the hybrids may show different methylation levels at these and other loci. Sites in which DNA methylation is not maintained may not represent the critical CpG residues involved in regulating imprinted-gene expression in this system. Alternatively, it is possible that the particular chromatin state associated with maternally and paternally imprinted chromosomes, perhaps in concert with specific trans factors, is sufficient to maintain imprinted-gene expression. This would then be analogous to the maintenance of the developmental state of β-globin gene expression (16) discussed earlier.
It is important to note that this system is constrained by the limitations imposed by imprinted genes subject to temporal and/or tissue-specific regulation. Although we generally have met with success in assaying imprinted-gene expression, we were not able to consistently detect expression of the ZNF127 transcript, presumably because of low levels of expression and or mRNA instability in the cell types used to generate our hybrid cell lines. The corollary to this may also be true; that is, because of the relative sensitivity of RT-PCR, one may detect expression of a transcript in both maternal and paternal chromosome containing hybrids when in fact, expression is predominantly silenced on one chromosome. This type of “leaky” expression has been shown for p57KIP2 (6) and IMPT1/ORCTL2 (48, 49).
The fact that imprinted-gene expression is maintained in somatic-cell hybrids is important not only for its scientific utility but from an evolutionary point of view. Once replicated, the human chromosomes contained in the hybrid cell lines are remodeled using rodent proteins. The perpetuation of expression imprints strongly supports the existence of evolutionarily conserved factors involved in maintenance of genomic imprinting, perhaps including cis DNA elements and/or chromatin proteins, and is consistent with the maintenance of developmental globin-gene expression (16) and X chromosome inactivation (17) states in somatic-cell hybrids. One use of this system may be to analyze molecular mechanisms involved in the maintenance of genomic imprints in somatic cells; for example, by testing the efficacy of various chemicals to activate genes normally silenced by genomic imprinting. It has been shown that treatment of cells with 5-azacytidine can demethylate promoters and induce transcriptional activation (50), including reactivation of X-linked genes in somatic-cell hybrids containing a previously inactive X chromosome (51). Similarly, histone H4 acetylation has been associated with transcriptional activation (52), so that treatment of cell lines with sodium butyrate or Trichostatin A, inhibitors of histone deacetylase, also may have direct effects on imprinted gene activity (53).
At present, however, we envision this model system being most useful in rapidly determining the imprinted status of transcripts. This will be particularly true as the Human Genome Project identifies a large number of genes mapping within or near regions thought to be imprinted. A panel of somatic-cell hybrids containing a maternal or paternal homolog of each human chromosome suspected to contain imprinted genes would be an invaluable resource for such imprinting assays.
Acknowledgments
We thank Dr. Huntington F. Willard for cell lines, as well as for critical input on experimental design and writing of this manuscript. We also thank Dr. Andrew Miller for mapping the RPS12 gene to chromosome 15, Stacey Bolk for help with microsatellite analysis, Dr. Bryan Williams for primers to WT1, James Amos-Landgraf for technical assistance, and Dr. Roger Schultz for cell line A15. This research was supported by the National Institutes of Health (Grant HD31491 to R.D.N. and CA63333 to T.B.S. and M.J.H.) and Pew Scholars Program in Biomedical Sciences (R.D.N.). J.M.G. was supported by National Institutes of Health Training Grant GM08613.
ABBREVIATIONS
- AS
Angelman syndrome
- PWS
Prader–Willi syndrome
- RT-PCR
reverse transcription–PCR
- UPD
uniparental disomy
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Solter D. Annu Rev Genet. 1988;22:127–146. doi: 10.1146/annurev.ge.22.120188.001015. [DOI] [PubMed] [Google Scholar]
- 2.Cattanach, B. M. & Beechey, C. V. (1990) Development (Cambridge, U.K.) Suppl. 63–72. [PubMed]
- 3.Nicholls R D, Saitoh S, Horsthemke B. Trends Genet. 1998;14:194–200. doi: 10.1016/s0168-9525(98)01432-2. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich B, Buiting K, Korn B, Rickard S, Buxton J, Saitoh S, Nicholls R D, Poustka A, Winterpacht A, Zabel B, et al. Nat Genet. 1996;14:163–170. doi: 10.1038/ng1096-163. [DOI] [PubMed] [Google Scholar]
- 5.Malzac P, Webber H, Moncla A, Graham J M, Kukolich M, Williams C, Pagon R A, Ramsdell L A, Kishino T, Wagstaff J. Am J Hum Genet. 1998;62:1353–1360. doi: 10.1086/301877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reik W, Maher E R. Trends Genet. 1997;13:330–334. doi: 10.1016/s0168-9525(97)01200-6. [DOI] [PubMed] [Google Scholar]
- 7.Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, et al. Nat Genet. 1996;14:171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- 8.Kondo M, Matsuoka S, Uchida K, Osada H, Nagatake M, Takagi K, Harper J W, Takahashi T, Elledge S, Takahashi T. Oncogene. 1996;12:1365–1368. [PubMed] [Google Scholar]
- 9.Thompson J S, Reese K J, DeBaun M R, Perlman E J, Feinberg A P. Cancer Res. 1996;56:5723–5727. [PubMed] [Google Scholar]
- 10.Hatada I, Inazawa J, Abe T, Nakayama M, Kaneko Y, Jinno Y, Niikawa N, Ohashi H, Fukushima Y, Iida K, et al. Hum Mol Genet. 1996;5:783–788. doi: 10.1093/hmg/5.6.783. [DOI] [PubMed] [Google Scholar]
- 11.Ledbetter D H, Engel E. Hum Mol Genet. 1995;4:1757–1764. doi: 10.1093/hmg/4.suppl_1.1757. [DOI] [PubMed] [Google Scholar]
- 12.Kuroiwa Y, Kaneko-Ishino T, Kagitani F, Kohda T, Li L L, Tada M, Suzuki R, Yokoyama M, Shiroishi T, Wakana S, et al. Nat Genet. 1996;12:186–190. doi: 10.1038/ng0296-186. [DOI] [PubMed] [Google Scholar]
- 13.Plass C, Shibata H, Kalcheva I, Mullins L, Kotelevtseva N, Mullins J, Kato R, Sasaki H, Hirotsune S, Okazaki Y, et al. Nat Genet. 1996;14:106–109. doi: 10.1038/ng0996-106. [DOI] [PubMed] [Google Scholar]
- 14.Hayashizaki Y, Shibata H, Hirotsune S, Sugino H, Okazaki Y, Sasaki N, Hirose K, Imoto H, Okuizumi H, Muramatsu M, et al. Nat Genet. 1994;6:33–40. doi: 10.1038/ng0194-33. [DOI] [PubMed] [Google Scholar]
- 15.Kalscheuer V M, Mariman E C, Schepens M T, Rehder H, Ropers H H. Nat Genet. 1993;5:74–78. doi: 10.1038/ng0993-74. [DOI] [PubMed] [Google Scholar]
- 16.Stanworth S J, Roberts N A, Sharpe J A, Sloane-Stanley J A, Wood W G. Mol Cell Biol. 1995;15:3969–3978. doi: 10.1128/mcb.15.8.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown C J, Carrel L, Willard H F. Am J Hum Genet. 1997;60:1333–1343. doi: 10.1086/515488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meguro M, Mitsuya K, Sui H, Shigenami K, Kugoh H, Nakao M, Oshimura M. Hum Mol Genet. 1997;6:2127–2133. doi: 10.1093/hmg/6.12.2127. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel L D, Schultz R A. Proc Natl Acad Sci USA. 1992;89:7968–7972. doi: 10.1073/pnas.89.17.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ning Y, Lovell M, Taylor L, Pereira-Smith O M. Cytogenet Cell Genet. 1992;60:79–80. doi: 10.1159/000133300. [DOI] [PubMed] [Google Scholar]
- 21.Glenn C C, Saitoh S, Jong M T C, Filbrandt M M, Surti U, Driscoll D J, Nicholls R D. Am J Hum Genet. 1996;58:335–346. [PMC free article] [PubMed] [Google Scholar]
- 22.Wevrick R, Kerns J A, Francke U. Hum Mol Genet. 1994;3:1877–1882. doi: 10.1093/hmg/3.10.1877. [DOI] [PubMed] [Google Scholar]
- 23.Sutcliffe J S, Nakao M, Christian S, Orstavik K H, Tommerup N, Ledbetter D H, Beaudet A L. Nat Genet. 1994;8:52–58. doi: 10.1038/ng0994-52. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Tycko B. Nat Genet. 1992;1:40–44. doi: 10.1038/ng0492-40. [DOI] [PubMed] [Google Scholar]
- 25.Giaclone J, Francke U. Hum Mol Genet. 1994;3:379. doi: 10.1093/hmg/3.2.379. [DOI] [PubMed] [Google Scholar]
- 26.Glenn C C, Porter K A, Jong M T C, Nicholls R D, Driscoll D J. Hum Mol Genet. 1993;12:2001–2005. doi: 10.1093/hmg/2.12.2001. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Nicholls R D, Butler M G, Saitoh S, Hainline B E, Palmer C G. Hum Mol Genet. 1996;5:517–524. doi: 10.1093/hmg/5.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solomon E, Bobrow M, Goodfellow P N, Bodmer W F, Swallow D M, Povey S, Noel B. Somatic Cell Genet. 1976;2:125–140. doi: 10.1007/BF01542626. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald H R, Wevrick R. Hum Mol Genet. 1997;6:1873–1878. doi: 10.1093/hmg/6.11.1873. [DOI] [PubMed] [Google Scholar]
- 30.Philippe J, Rougeulle C, Massacrier A, Moncla A, Mattei M-G, Malzac P, Roeckel N, Taviaux S, Lefranc J-L B, Cau P, et al. Nat Genet. 1997;17:357–361. doi: 10.1038/ng1197-357. [DOI] [PubMed] [Google Scholar]
- 31.Dittrich B, Buiting K, Grob S, Horsthemke B. Hum Mol Genet. 1993;12:1995–1999. doi: 10.1093/hmg/2.12.1995. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls R D, Gottlieb W, Russell L B, Davda M, Horsthemke B, Rinchik E M. Proc Natl Acad Sci USA. 1993;90:2050–2054. doi: 10.1073/pnas.90.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culiat C T, Stubbs L J, Montgomery C S, Russell L B, Rinchik E M. Proc Natl Acad Sci USA. 1994;91:2815–2818. doi: 10.1073/pnas.91.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homanics G E, DeLorey T M, Firestone L L, Quinlan J J, Handforth A, Harrison N L, Krasowski M D, Rick C E, Korpi E R, Makela R, et al. Proc Natl Acad Sci USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannoukakis N, Cheri D, Paquette J, Goodyer C G, Polychronakos C. Nat Genet. 1993;4:98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- 36.Adam G I, Cui H, Miller S J, Flam F, Ohlsson R. Development (Cambridge, UK) 1996;122:839–847. doi: 10.1242/dev.122.3.839. [DOI] [PubMed] [Google Scholar]
- 37.Brown K W, Villar A J, Bickmore W, Clayton-Smith J, Catchpoole D, Maher E R, Reik W. Hum Mol Genet. 1996;5:2027–2032. doi: 10.1093/hmg/5.12.2027. [DOI] [PubMed] [Google Scholar]
- 38.Caspary T, Cleary M A, Baker C C, Guan X J, Tilghman S M. Mol Cell Biol. 1998;18:3466–3474. doi: 10.1128/mcb.18.6.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Little M H. Oncogene. 1992;7:635–641. [PubMed] [Google Scholar]
- 40.Jinno Y, Yun K, Nishiwaki K, Kubota T, Ogawa O, Reeve A E, Niikawa N. Nat Genet. 1994;6:305–309. doi: 10.1038/ng0394-305. [DOI] [PubMed] [Google Scholar]
- 41.Reid L H, Davies C, Cooper P R, Crider-Miller S J, Sait S N, Nowak N J, Evans G, Stanbridge E J, de Jong P, Shows T B, et al. Genomics. 1997;43:366–375. doi: 10.1006/geno.1997.4826. [DOI] [PubMed] [Google Scholar]
- 42.Maecker H T, Do M S, Levy S. Proc Natl Acad Sci USA. 1998;95:2458–2462. doi: 10.1073/pnas.95.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aizawa T, Maruyama K, Kondo H, Yoshikawa K. Brain Res Dev Brain Res. 1992;68:265–274. doi: 10.1016/0165-3806(92)90069-9. [DOI] [PubMed] [Google Scholar]
- 44.Kubota T, Niikawa N, Jinno Y, Ishimura T. Am J Med Genet. 1994;49:452–453. doi: 10.1002/ajmg.1320490422. [DOI] [PubMed] [Google Scholar]
- 45.Mutter G L, Stewart C L, Chaponot M L, Pomponio R J. Am J Hum Genet. 1993;53:1096–1102. [PMC free article] [PubMed] [Google Scholar]
- 46.Mowery-Rushton P A, Driscoll D J, Nicholls R D, Joseph L, Urvashi S. Am J Hum Genet. 1996;61:140–146. doi: 10.1002/(SICI)1096-8628(19960111)61:2<140::AID-AJMG7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Culiat C T, Stubbs L, Woychik R P, Russell L B, Johnson D K, Rinchik E M. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- 48.Dao D, Frank D, Qian N, O’Keefe D, Vosatka R J, Walsh C P, Tycko B. Hum Mol Genet. 1998;7:597–608. doi: 10.1093/hmg/7.4.597. [DOI] [PubMed] [Google Scholar]
- 49.Cooper P R, Smilinich N J, Day C D, Nowak N J, Reid L H, Pearsall R S, Reece M, Prawitt D, Landers J, Housman D E, et al. Genomics. 1998;49:38–51. doi: 10.1006/geno.1998.5221. [DOI] [PubMed] [Google Scholar]
- 50.Robertson K D, Hayward D, Ling P D, Samid D, Ambinder R F. Mol Cell Biol. 1995;15:6150–6159. doi: 10.1128/mcb.15.11.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohandas T, Sparkes R S, Shapiro L J. Science. 1981;211:393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- 52.Loidl P. Chromosoma. 1994;103:441–449. doi: 10.1007/BF00337382. [DOI] [PubMed] [Google Scholar]
- 53.Jeppeson P, Turner B M. Cell. 1993;74:281–289. doi: 10.1016/0092-8674(93)90419-q. [DOI] [PubMed] [Google Scholar]
- 54.Brown C J, Powers V E, Munroe D L, Sheinin R, Willard H F. Somatic Cell Genet. 1989;15:173–178. doi: 10.1007/BF01535079. [DOI] [PubMed] [Google Scholar]
- 55.Shows T B, Brown J A. Proc Natl Acad Sci USA. 1975;72:2125–2129. doi: 10.1073/pnas.72.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider R, Higgins M J, Kieninger D, Schneider-Scherzer E, Hirsch- Kauffmann M, Schweiger M, Eddy R L, Shows T B, Zabal B U. Cytogenet Cell Genet. 1992;59:264–267. doi: 10.1159/000133265. [DOI] [PubMed] [Google Scholar]
- 57.Brown C J, Willard H F. Nature (London) 1994;368:646–648. doi: 10.1038/368154a0. [DOI] [PubMed] [Google Scholar]