Abstract
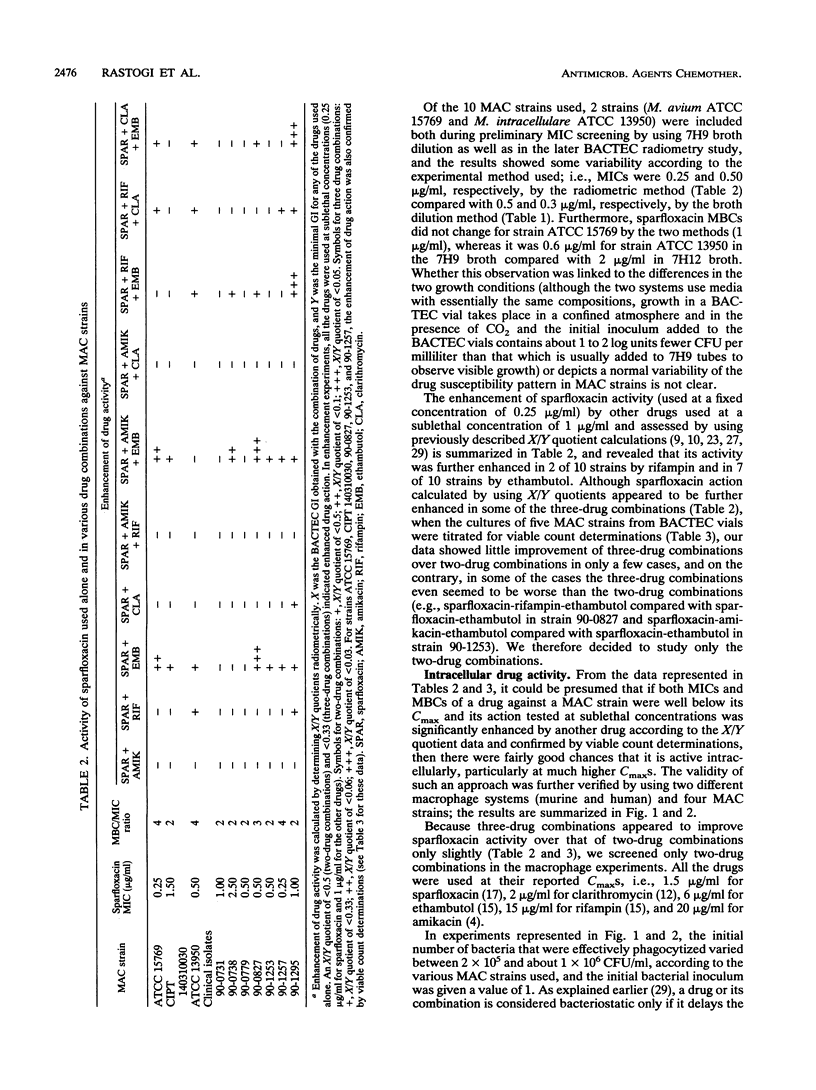
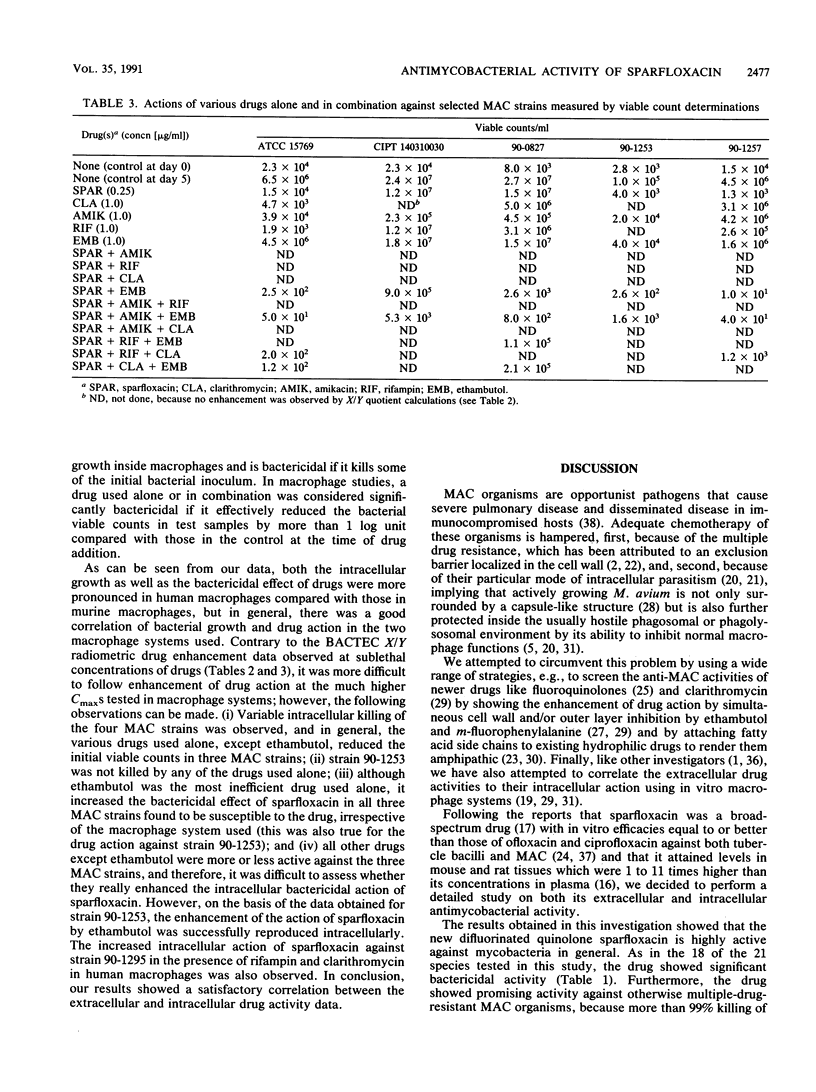
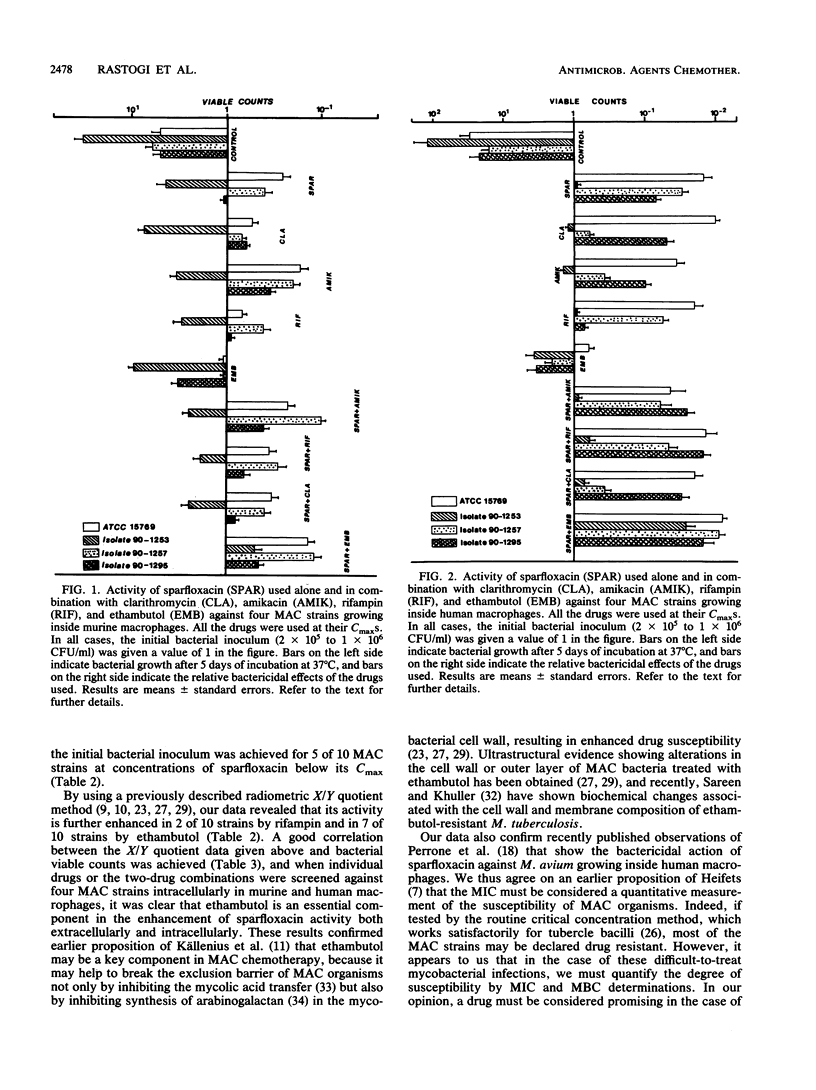
The MICs and MBCs of the new difluorinated quinolone drug sparfloxacin against type strains belonging to 21 species of mycobacteria were screened. The MICs and MBCs were within the range of 0.1 to 2.0 and 0.1 to 4.0 micrograms/ml, respectively (with an MBC/MIC ratio of 1 to 2), and against 18 of the 21 species tested, the drug showed significant bactericidal activity (at least 99% killing or more of the initial inoculum added) at concentrations well within the reported peak concentrations in serum (Cmax) in humans. MICs of sparfloxacin for 7 of 10 Mycobacterium avium complex strains were below the Cmax, with MBC/MIC ratios within the range of 2 to 4. Enhancement of its activity by ethambutol, rifampin, amikacin, and clarithromycin (which were used at sublethal concentrations) assessed by using BACTEC radiometry revealed that its activity was further enhanced in 2 of 10 strains by rifampin and in 7 of 10 strains by ethambutol. The bactericidal effects of various drugs used alone as well as two-drug combinations used at Cmax levels were also screened against four strains of M. avium complex growing intracellularly in two different macrophage systems, namely, mouse bone marrow-derived macrophages and peripheral blood monocyte-derived human macrophages. Our results showed a satisfactory correlation between the extracellular and intracellular drug activity data.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J., Elkins N., May M. H. Effectiveness of ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium, and rifampin against M. tuberculosis in cultured human macrophages. Am Rev Respir Dis. 1988 May;137(5):1141–1146. doi: 10.1164/ajrccm/137.5.1141. [DOI] [PubMed] [Google Scholar]
- David H. L., Rastogi N., Clavel-Sérès S., Clément F., Thorel M. F. Structure of the cell envelope of Mycobacterium avium. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Apr;264(1-2):49–66. doi: 10.1016/s0176-6724(87)80124-4. [DOI] [PubMed] [Google Scholar]
- Davies S., Sparham P. D., Spencer R. C. Comparative in-vitro activity of five fluoroquinolones against mycobacteria. J Antimicrob Chemother. 1987 May;19(5):605–609. doi: 10.1093/jac/19.5.605. [DOI] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Hitt J. A. Tissue penetration of the new quinolones in humans. Rev Infect Dis. 1989 Jul-Aug;11 (Suppl 5):S1046–S1057. doi: 10.1093/clinids/11.supplement_5.s1046. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Ethambutol MICs and MBCs for Mycobacterium avium complex and Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1986 Dec;30(6):927–932. doi: 10.1128/aac.30.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. MIC as a quantitative measurement of the susceptibility of Mycobacterium avium strains to seven antituberculosis drugs. Antimicrob Agents Chemother. 1988 Aug;32(8):1131–1136. doi: 10.1128/aac.32.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffner S. E., Källenius G., Beezer A. E., Svenson S. B. Studies on the mechanisms of the synergistic effects of ethambutol and other antibacterial drugs on Mycobacterium avium complex. Acta Leprol. 1989;7 (Suppl 1):195–199. [PubMed] [Google Scholar]
- Hoffner S. E., Svenson S. B., Källenius G. Synergistic effects of antimycobacterial drug combinations on Mycobacterium avium complex determined radiometrically in liquid medium. Eur J Clin Microbiol. 1987 Oct;6(5):530–535. doi: 10.1007/BF02014241. [DOI] [PubMed] [Google Scholar]
- Killing intracellular mycobacteria: dogmas and realities. 5th Forum in Microbiology. Proceedings. Res Microbiol. 1990 Feb;141(2):191–270. doi: 10.1016/0923-2508(90)90029-p. [DOI] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother. 1989 Sep;33(9):1419–1422. doi: 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Dec;31(12):1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Svenson S. B., Hoffner S. E. Ethambutol: a key for Mycobacterium avium complex chemotherapy? Am Rev Respir Dis. 1989 Jul;140(1):264–264. doi: 10.1164/ajrccm/140.1.264. [DOI] [PubMed] [Google Scholar]
- Leysen D. C., Haemers A., Pattyn S. R. Mycobacteria and the new quinolones. Antimicrob Agents Chemother. 1989 Jan;33(1):1–5. doi: 10.1128/aac.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kurobe N., Ohue T., Hashimoto M., Shimizu M. Pharmacokinetics of a novel quinolone, AT-4140, in animals. Antimicrob Agents Chemother. 1990 Jan;34(1):89–93. doi: 10.1128/aac.34.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Nakata K., Kurobe N., Kouno K., Sakaguchi Y., Kashimoto S., Yoshida H., Kojima T., Ohue T. In vitro and in vivo antibacterial activities of AT-4140, a new broad-spectrum quinolone. Antimicrob Agents Chemother. 1989 Aug;33(8):1167–1173. doi: 10.1128/aac.33.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Vilde J. L., Pocidalo J. J. Activities of sparfloxacin, azithromycin, temafloxacin, and rifapentine compared with that of clarithromycin against multiplication of Mycobacterium avium complex within human macrophages. Antimicrob Agents Chemother. 1991 Jul;35(7):1356–1359. doi: 10.1128/aac.35.7.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Blom-Potar M. C. Intracellular bactericidal activity of ciprofloxacin and ofloxacin against Mycobacterium tuberculosis H37Rv multiplying in the J-774 macrophage cell line. Zentralbl Bakteriol. 1990 Jun;273(2):195–199. doi: 10.1016/s0934-8840(11)80249-5. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S. Action of 1-isonicotinyl-2-palmitoyl hydrazine against the Mycobacterium avium complex and enhancement of its activity by m-fluorophenylalanine. Antimicrob Agents Chemother. 1990 Nov;34(11):2061–2064. doi: 10.1128/aac.34.11.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Activity of five fluoroquinolones against Mycobacterium avium-intracellulare complex and M. xenopi. Ann Inst Pasteur Microbiol. 1988 Mar-Apr;139(2):233–237. doi: 10.1016/0769-2609(88)90008-7. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Drug susceptibility testing in tuberculosis: a comparison of the proportion methods using Lowenstein-Jensen, Middlebrook 7H10 and 7H11 agar media and a radiometric method. Res Microbiol. 1989 Jul-Aug;140(6):405–417. doi: 10.1016/0923-2508(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Enhancement of drug susceptibility of Mycobacterium avium by inhibitors of cell envelope synthesis. Antimicrob Agents Chemother. 1990 May;34(5):759–764. doi: 10.1128/aac.34.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S. In vitro activity of the new difluorinated quinolone sparfloxacin (AT-4140) against Mycobacterium tuberculosis compared with activities of ofloxacin and ciprofloxacin. Antimicrob Agents Chemother. 1991 Sep;35(9):1933–1936. doi: 10.1128/aac.35.9.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Hellio R. Evidence that the capsule around mycobacteria grown in axenic media contains mycobacterial antigens: implications at the level of cell envelope architecture. FEMS Microbiol Lett. 1990 Jul;58(2):161–166. doi: 10.1111/j.1574-6968.1990.tb13971.x. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V. Extracellular and intracellular activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Mar;35(3):462–470. doi: 10.1128/aac.35.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Moreau B., Capmau M. L., Goh K. S., David H. L. Antibacterial action of amphipathic derivatives of isoniazid against the Mycobacterium avium complex. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jun;268(4):456–462. doi: 10.1016/s0176-6724(88)80123-8. [DOI] [PubMed] [Google Scholar]
- Sareen M., Khuller G. K. Cell wall and membrane changes associated with ethambutol resistance in Mycobacterium tuberculosis H37Ra. Antimicrob Agents Chemother. 1990 Sep;34(9):1773–1776. doi: 10.1128/aac.34.9.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Armstrong E. L., Kunugi K. A., Kilburn J. O. Inhibition by ethambutol of mycolic acid transfer into the cell wall of Mycobacterium smegmatis. Antimicrob Agents Chemother. 1979 Aug;16(2):240–242. doi: 10.1128/aac.16.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Kilburn J. O. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989 Sep;33(9):1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Matsumoto T., Husson M. Intraphagocytic penetration of antibiotics. J Antimicrob Chemother. 1988 Aug;22(2):185–192. doi: 10.1093/jac/22.2.185. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Sanders C. A., Hadley W. K. Killing by antimycobacterial agents of AIDS-derived strains of Mycobacterium avium complex inside cells of the mouse macrophage cell line J774. Am Rev Respir Dis. 1989 Nov;140(5):1198–1203. doi: 10.1164/ajrccm/140.5.1198. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Sanders C. A., Nassos P. S., Hadley W. K. In vitro susceptibility of Mycobacterium avium complex to the new fluoroquinolone sparfloxacin (CI-978; AT-4140) and comparison with ciprofloxacin. Antimicrob Agents Chemother. 1990 Dec;34(12):2442–2444. doi: 10.1128/aac.34.12.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]


