Abstract
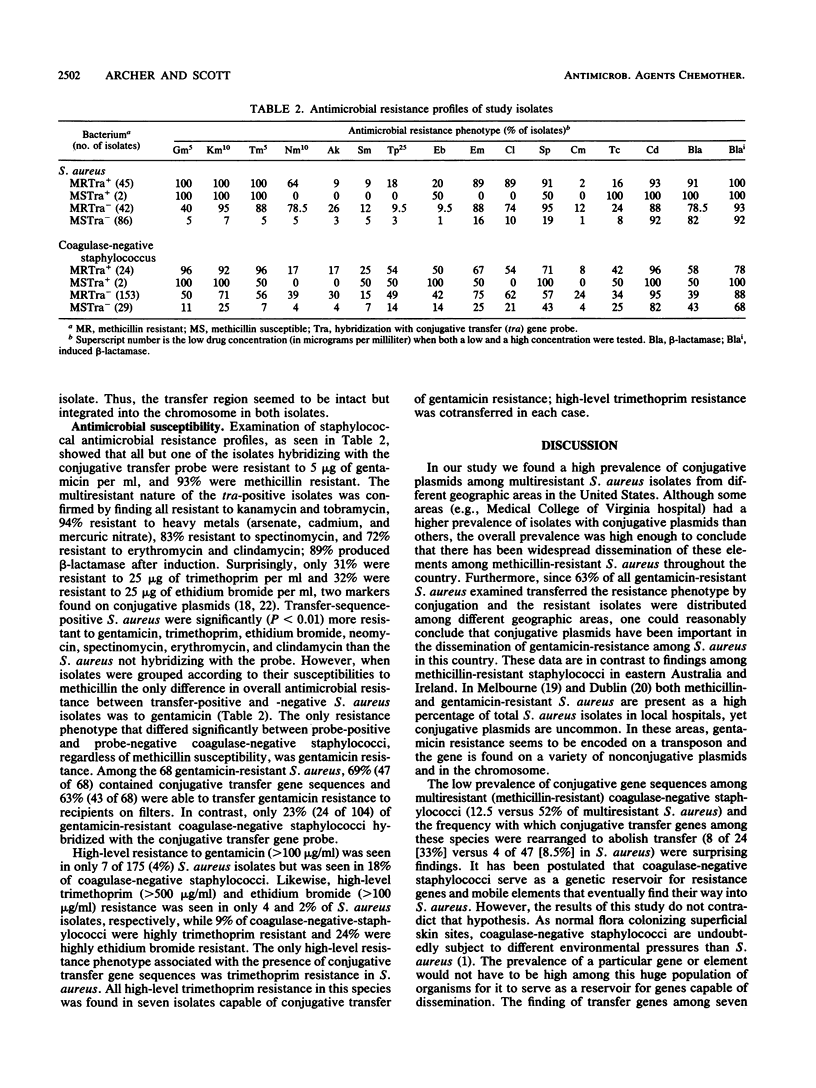
Staphylococcus aureus and coagulase-negative staphylococcal isolates from various geographic areas in the United States were examined by using a conjugative transfer gene DNA probe in dot-blot hybridization assays. Of 175 S. aureus isolates, 47 (27%) hybridized with the probe, while 24 of 208 (11.5%) coagulase-negative staphylococci hybridized. However, among methicillin-resistant S. aureus 52% (45 of 89) were probe positive while only 2% (2 of 86) of methicillin-susceptible S. aureus were probe positive. In contrast, 12.5% (22 of 176) of methicillin-resistant and 6% (2 of 32) of methicillin-susceptible coagulase-negative staphylococci contained transfer genes. All but one of the staphylococci containing transfer genes were resistant to gentamicin; 91.5% of S. aureus and 65% of coagulase-negative staphylococci containing transfer genes transferred gentamicin resistance to a S. aureus recipient. Of the 12 isolates that hybridized with the probe but did not transfer resistance, 10 (6 coagulase-negative staphylococci and 4 S. aureus) carried both gentamicin resistance and conjugative transfer genes on the same plasmid. Of these 10, 6 contained plasmid target fragments of sizes different from that of the probe, suggesting additions or deletions of DNA essential for transfer, while in 4 no such alterations could be detected. In two coagulase-negative staphylococci the entire transfer region was apparently integrated into the chromosome. Thus, staphylococci carrying conjugative transfer genes are widely disseminated in the United States and are usually found in multiresistant isolates on plasmids that also encode gentamicin resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Karchmer A. W., Vishniavsky N., Johnston J. L. Plasmid-pattern analysis for the differentiation of infecting from noninfecting Staphylococcus epidermidis. J Infect Dis. 1984 Jun;149(6):913–920. doi: 10.1093/infdis/149.6.913. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Pennell E. Detection of methicillin resistance in staphylococci by using a DNA probe. Antimicrob Agents Chemother. 1990 Sep;34(9):1720–1724. doi: 10.1128/aac.34.9.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes B. A., Schaberg D. R. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983 Feb;153(2):627–634. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto D. W., Johnston J. L., Archer G. L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 Nov;31(11):1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M. T., Lyon B. R., Skurray R. A. Typing of methicillin-resistant Staphylococcus aureus by antibiotic resistance phenotypes. J Med Microbiol. 1990 Jan;31(1):57–64. doi: 10.1099/00222615-31-1-57. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Gardiner R. V., Packer R. R. The prevalence of staphylococcal resistance to penicillinase-resistant penicillins. A retrospective and prospective national surveillance trial of isolates from 40 medical centers. Diagn Microbiol Infect Dis. 1989 Sep-Oct;12(5):385–394. doi: 10.1016/0732-8893(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Lee J. C., Alexander G. A. Rapid penicillinase paper strip test for detection of beta-lactamase-producing Haemophilus influenzae and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Jun;11(6):1087–1088. doi: 10.1128/aac.11.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos W. E., Schleifer K. H. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975 Jan;1(1):82–88. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell R. W., Sweeney H. M., Cohen S. Conjugational transfer of gentamicin resistance plasmids intra- and interspecifically in Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1983 Jan;23(1):151–160. doi: 10.1128/aac.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S. J., Archer G. L. Mobilization of the relaxable Staphylococcus aureus plasmid pC221 by the conjugative plasmid pGO1 involves three pC221 loci. J Bacteriol. 1989 Apr;171(4):1841–1845. doi: 10.1128/jb.171.4.1841-1845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch D. A., Messerotti L. J., Loo L. S., Jackson C. A., Skurray R. A. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol Microbiol. 1989 Feb;3(2):161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Power G., Betzold J., Forbes B. A. Conjugative R plasmids in antimicrobial resistance of Staphylococcus aureus causing nosocomial infections. J Infect Dis. 1985 Jul;152(1):43–49. doi: 10.1093/infdis/152.1.43. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Rouch D. A., Lyon B. R., Gillespie M. T., Tennent J. M., Byrne M. E., Messerotti L. J., May J. W. Multiresistant Staphylococcus aureus: genetics and evolution of epidemic Australian strains. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):19–39. doi: 10.1093/jac/21.suppl_c.19. [DOI] [PubMed] [Google Scholar]
- Storrs M. J., Courvalin P., Foster T. J. Genetic analysis of gentamicin resistance in methicillin- and gentamicin-resistant strains of Staphylococcus aureus isolated in Dublin hospitals. Antimicrob Agents Chemother. 1988 Aug;32(8):1174–1181. doi: 10.1128/aac.32.8.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989 Feb;171(2):684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Mobility of gentamicin resistance genes from staphylococci isolated in the United States: identification of Tn4031, a gentamicin resistance transposon from Staphylococcus epidermidis. Antimicrob Agents Chemother. 1989 Aug;33(8):1335–1341. doi: 10.1128/aac.33.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R. A., Kabins S. A., Nathan C., Sweeney H. M., Jaffe H. W., Cohen S. Gentamicin-resistant staphylococci as hospital flora: epidemiology and resistance plasmids. J Infect Dis. 1982 Mar;145(3):374–382. doi: 10.1093/infdis/145.3.374. [DOI] [PubMed] [Google Scholar]


