Abstract
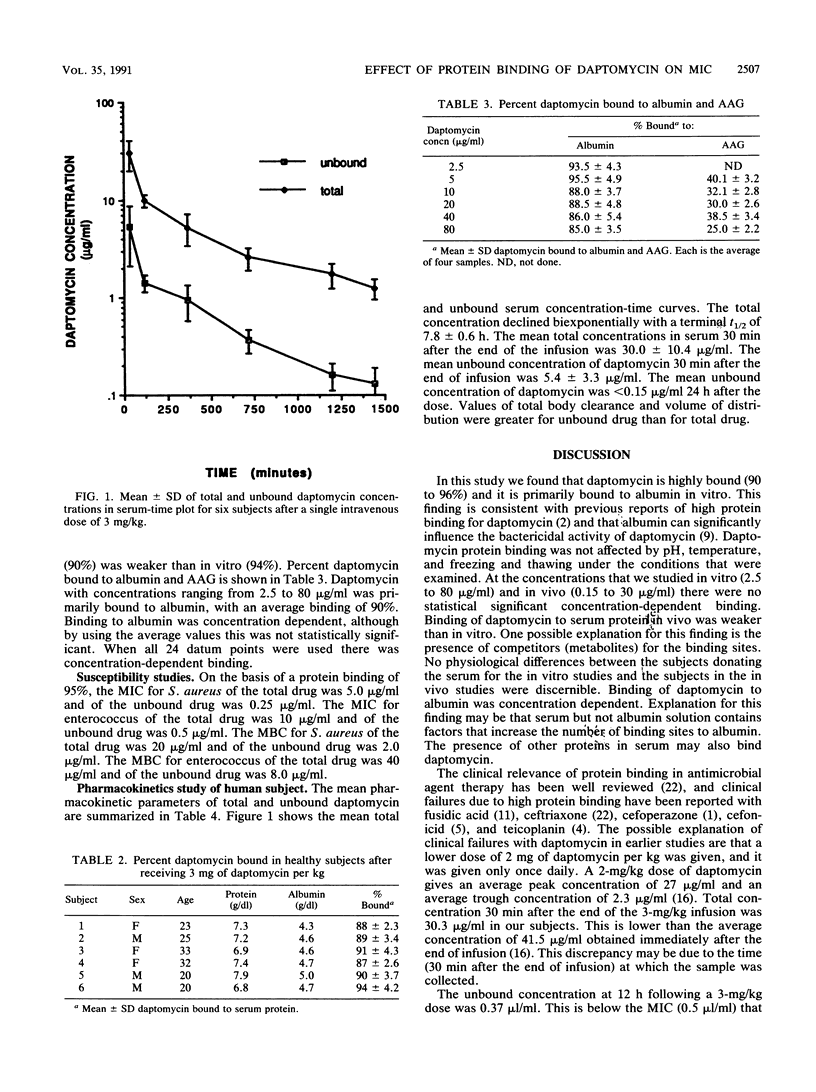
A higher rate of clinical failures in patients treated with daptomycin (2 mg/kg of body weight, given once daily) compared with rates in patients treated with conventional regimens caused early termination of this comparative clinical trial. One explanation for these failures could be that daptomycin is highly protein bound and that the concentration of the unbound active drug is too low for antibacterial activity. To assess this explanation, we studied the binding of daptomycin to proteins by using an ultrafiltration method. pH (7.0 to 7.4), temperature (25 or 37 degrees C), or daily freezing and thawing over 2 months had no effect on binding of daptomycin to proteins. We found that daptomycin was bound to albumin (90%) at 4 g/100 ml. Binding of daptomycin was not concentration dependent (2.5 to 80 micrograms/ml). In human serum samples spiked with daptomycin, average binding was 94% +/- 2.4%. In 6 subjects given an intravenous infusion of daptomycin (3 mg/kg), average binding was 90% +/- 2.1%. Susceptibility studies showed that a concentration in serum 20 times the unbound concentration was needed to equal the MIC of the total drug. These results indicate that daptomycin is highly bound (90 to 94%) to albumin and that clinical failure to daptomycin can in part be explained by the low concentration of the unbound drug.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar R., Fainstein V., Elting L., Bodey G. P. Cefoperazone for the treatment of infections in patients with cancer. Rev Infect Dis. 1983 Mar-Apr;5 (Suppl 1):S181–S187. doi: 10.1093/clinids/5.supplement_1.s181. [DOI] [PubMed] [Google Scholar]
- Bush L. M., Boscia J. A., Kaye D. Daptomycin (LY146032) treatment of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1988 Jun;32(6):877–881. doi: 10.1128/aac.32.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calain P., Krause K. H., Vaudaux P., Auckenthaler R., Lew D., Waldvogel F., Hirschel B. Early termination of a prospective, randomized trial comparing teicoplanin and flucloxacillin for treating severe staphylococcal infections. J Infect Dis. 1987 Feb;155(2):187–191. doi: 10.1093/infdis/155.2.187. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Mills J., Drake T. A., Sande M. A. Failure of a once-daily regimen of cefonicid for treatment of endocarditis due to Staphylococcus aureus. Rev Infect Dis. 1984 Nov-Dec;6 (Suppl 4):S870–S874. doi: 10.1093/clinids/6.supplement_4.s870. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Willey S., Reiszner E., Spitzer P. G., Caputo G., Moellering R. C., Jr In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986 Oct;30(4):532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro activity of LY146032 against staphylococci, streptococci, and enterococci. Antimicrob Agents Chemother. 1986 Nov;30(5):781–784. doi: 10.1128/aac.30.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison M. W., Vance-Bryan K., Larson T. A., Toscano J. P., Rotschafer J. C. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1990 Oct;34(10):1925–1931. doi: 10.1128/aac.34.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güttler F., Tybring L., Engberg-Pedersen H. Interaction of albumin and fusidic acid. Br J Pharmacol. 1971 Sep;43(1):151–160. doi: 10.1111/j.1476-5381.1971.tb07164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob Agents Chemother. 1987 Apr;31(4):625–629. doi: 10.1128/aac.31.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin C. M., Craig W. A., Kornguth M., Monson R. Influence of binding on the pharmacologic activity of antibiotics. Ann N Y Acad Sci. 1973 Nov 26;226:214–224. doi: 10.1111/j.1749-6632.1973.tb20483.x. [DOI] [PubMed] [Google Scholar]
- Lam Y. W., Duroux M. H., Gambertoglio J. G., Barriere S. L., Guglielmo B. J. Effect of protein binding on serum bactericidal activities of ceftazidime and cefoperazone in healthy volunteers. Antimicrob Agents Chemother. 1988 Mar;32(3):298–302. doi: 10.1128/aac.32.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P. J., Gibaldi M., Stoeckel K. Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding. I. Theoretical considerations. Eur J Clin Pharmacol. 1983;25(3):399–405. doi: 10.1007/BF01037955. [DOI] [PubMed] [Google Scholar]
- Merrikin D. J., Briant J., Rolinson G. N. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983 Mar;11(3):233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urien S., Albengres E., Pinquier J. L., Tillement J. P. Role of alpha-1 acid glycoprotein, albumin, and nonesterified fatty acids in serum binding of apazone and warfarin. Clin Pharmacol Ther. 1986 Jun;39(6):683–689. doi: 10.1038/clpt.1986.119. [DOI] [PubMed] [Google Scholar]
- Wise R. The clinical relevance of protein binding and tissue concentrations in antimicrobial therapy. Clin Pharmacokinet. 1986 Nov-Dec;11(6):470–482. doi: 10.2165/00003088-198611060-00004. [DOI] [PubMed] [Google Scholar]


