Abstract
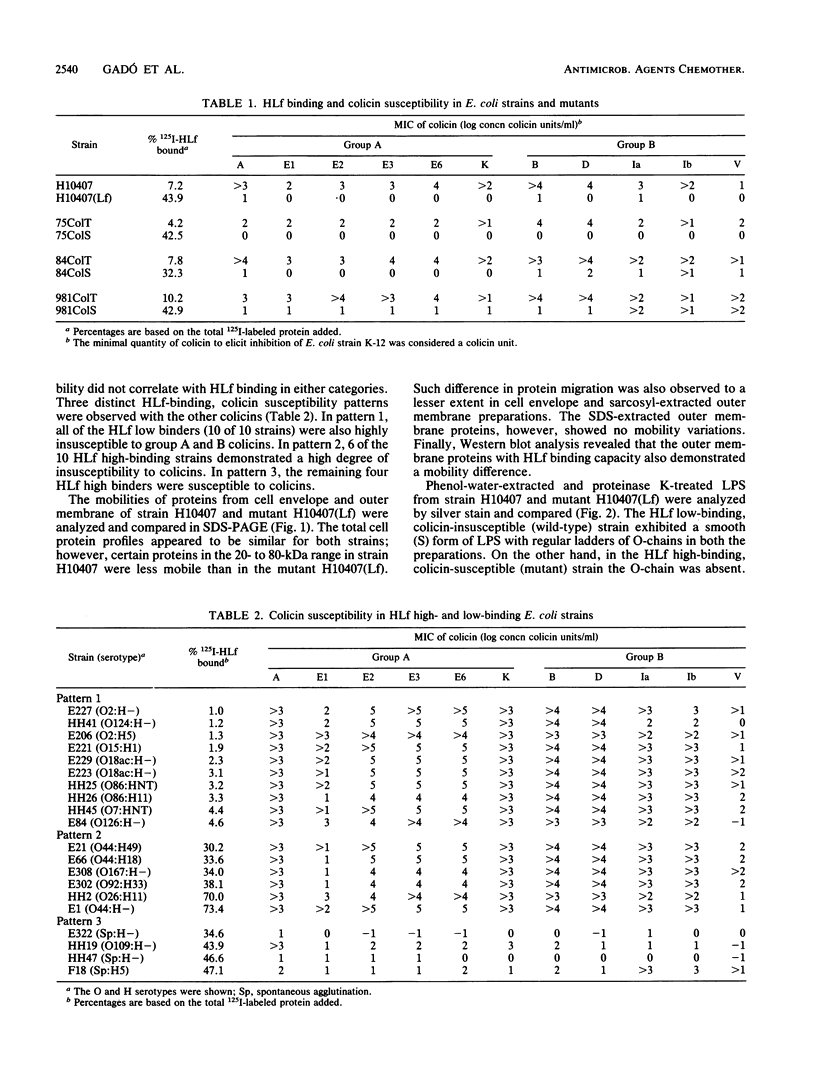
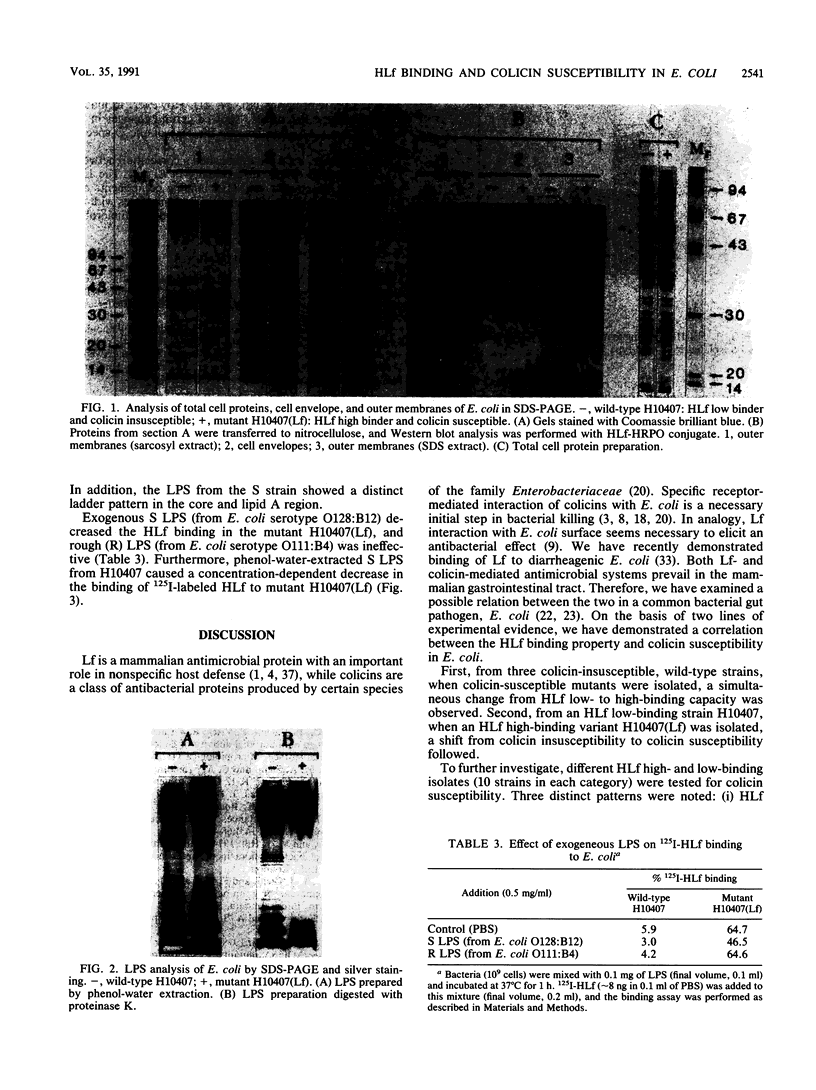
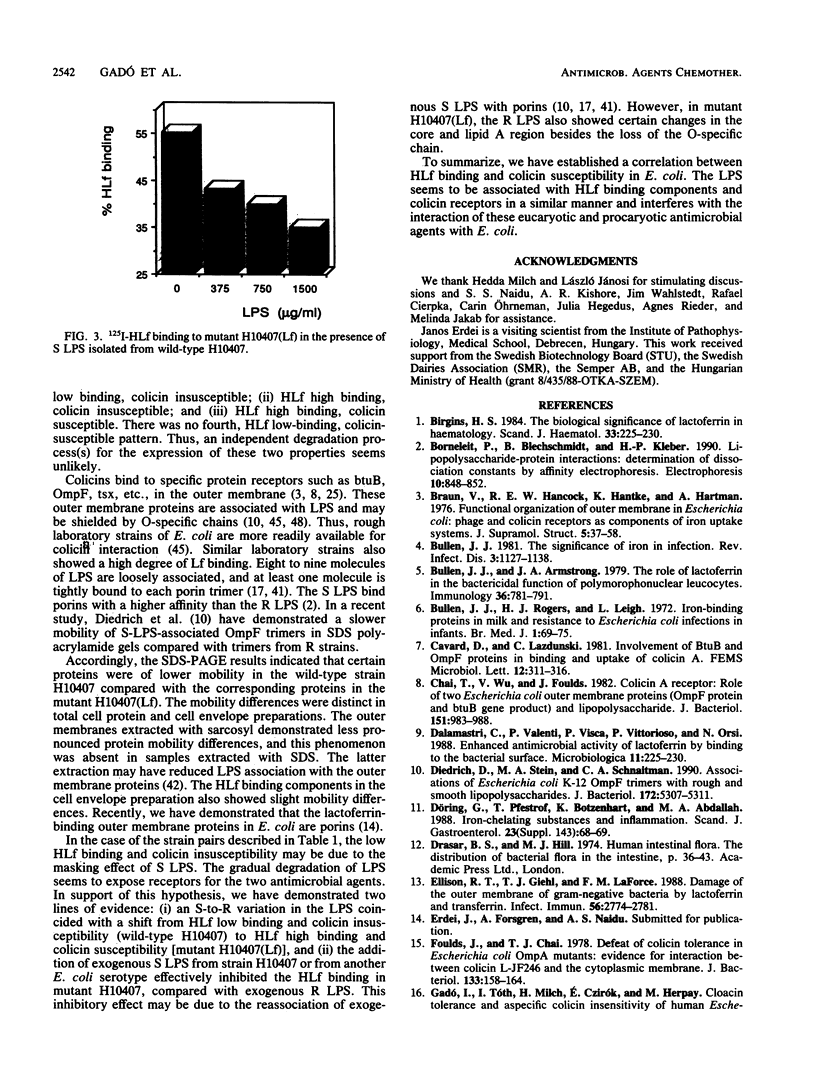
Escherichia coli H10407 demonstrated low 125I-human lactoferrin (HLf) binding (7%) and was insusceptible to group A (A, E1, E2, E3, E6, and K) and group B (B, D, Ia, Ib, and V) colicins. Conversely, a spontaneous HLf high-binding (44%) variant, H10407(Lf), demonstrated an increase susceptibility to both colicin groups. Colicin-insusceptible E. coli wild-type strains 75ColT, 84ColT, and 981ColT showed a low degree of HLf binding, i.e., 4, 8, and 10%, respectively. The HLf binding capacity was high in the corresponding colicin-susceptible mutants 75ColS (43%), 84ColS (32%), and 981ColS (43%). Furthermore, HLf low- (less than 5%) and high- (greater than 35%) binding E. coli clinical isolates (10 in each category) were tested for susceptibility against 11 colicins. Colicin V susceptibility did not correlate with HLf binding in either categories. However, with the remaining colicins, three distinct HLf-binding, colicin susceptibility patterns were observed; (i) 10 of 10 HLf low-binding strains were colicin insusceptible, (ii) 6 of 10 HLf high-binding strains were also colicin insusceptible, and (iii) the remaining HLf high binders were highly colicin susceptible. Certain proteins in the cell envelope and outer membrane of wild-type H10407 (HLf low binder, colicin insusceptible) showed a lower mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis compared to the corresponding proteins of mutant H10407(Lf) (HLf high binder, colicin susceptible). These mobility differences were also associated with HLf-binding proteins in Western blot (ligand blot) analysis. The wild type showed a smooth form of lipopolysaccharide (LPS) with a distinct ladder of O-chains, compared to the rough LPS of the mutant.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birgens H. S. The biological significance of lactoferrin in haematology. Scand J Haematol. 1984 Sep;33(3):225–230. doi: 10.1111/j.1600-0609.1984.tb02220.x. [DOI] [PubMed] [Google Scholar]
- Borneleit P., Blechschmidt B., Kleber H. P. Lipopolysaccharide-protein interactions: determination of dissociation constants by affinity electrophoresis. Electrophoresis. 1989 Dec;10(12):848–852. doi: 10.1002/elps.1150101209. [DOI] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Armstrong J. A. The role of lactoferrin in the bactericidal function of polymorphonuclear leucocytes. Immunology. 1979 Apr;36(4):781–791. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Chai T., Wu V., Foulds J. Colicin A receptor: role of two Escherichia coli outer membrane proteins (OmpF protein and btuB gene product) and lipopolysaccharide. J Bacteriol. 1982 Aug;151(2):983–988. doi: 10.1128/jb.151.2.983-988.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmastri C., Valenti P., Visca P., Vittorioso P., Orsi N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica. 1988 Jul;11(3):225–230. [PubMed] [Google Scholar]
- Diedrich D. L., Stein M. A., Schnaitman C. A. Associations of Escherichia coli K-12 OmpF trimers with rough and smooth lipopolysaccharides. J Bacteriol. 1990 Sep;172(9):5307–5311. doi: 10.1128/jb.172.9.5307-5311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J., LaForce F. M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988 Nov;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Chai T. J. Defeat of colicin tolerance in Escherichia coli ompA mutants: evidence for interaction between colicin L-JF246 and the cytoplasmic membrane. J Bacteriol. 1978 Jan;133(1):158–164. doi: 10.1128/jb.133.1.158-164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenburg A., Engel A., Kessler R., Manz H. J., Lustig A., Aebi U. Rapid isolation of OmpF porin-LPS complexes suitable for structure-function studies. Biochemistry. 1989 May 16;28(10):4187–4193. doi: 10.1021/bi00436a010. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Bassford P. J., Jr, Pugsley A. P. Colicin receptors and the mechanisms of colicin uptake. Zentralbl Bakteriol Orig A. 1979 Jun;244(1):90–104. [PubMed] [Google Scholar]
- Kishore A. R., Erdei J., Naidu S. S., Falsen E., Forsgren A., Naidu A. S. Specific binding of lactoferrin to Aeromonas hydrophila. FEMS Microbiol Lett. 1991 Sep 15;67(1):115–119. doi: 10.1016/0378-1097(91)90454-i. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Lactoferrin effects on phagocytic cell function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985 Jun;134(6):4176–4183. [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milch H., Nikolnikov S., Czirók E. Escherichia coli Col V plasmids and their role in pathogenicity. Acta Microbiol Hung. 1984;31(2):117–125. [PubMed] [Google Scholar]
- Naidu A. S., Andersson M., Miedzobrodzki J., Forsgren A., Watts J. L. Bovine lactoferrin receptors in Staphylococcus aureus isolated from bovine mastitis. J Dairy Sci. 1991 Apr;74(4):1218–1226. doi: 10.3168/jds.s0022-0302(91)78277-5. [DOI] [PubMed] [Google Scholar]
- Naidu A. S., Miedzobrodzki J., Andersson M., Nilsson L. E., Forsgren A., Watts J. L. Bovine lactoferrin binding to six species of coagulase-negative staphylococci isolated from bovine intramammary infections. J Clin Microbiol. 1990 Oct;28(10):2312–2319. doi: 10.1128/jcm.28.10.2312-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A. S., Miedzobrodzki J., Musser J. M., Rosdahl V. T., Hedström S. A., Forsgren A. Human lactoferrin binding in clinical isolates of Staphylococcus aureus. J Med Microbiol. 1991 Jun;34(6):323–328. doi: 10.1099/00222615-34-6-323. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part I: Production, and translocation across membranes. Microbiol Sci. 1984 Oct;1(7):168–175. [PubMed] [Google Scholar]
- Rainard P. Bacteriostasis of Escherichia coli by bovine lactoferrin, transferrin and immunoglobulins (IgG1, IgG2, IgM) acting alone or in combination. Vet Microbiol. 1986 Feb;11(1-2):103–115. doi: 10.1016/0378-1135(86)90011-8. [DOI] [PubMed] [Google Scholar]
- Reiter B., Brock J. H., Steel E. D. Inhibition of Escherichia coli by bovine colostrum and post-colostral milk. II. The bacteriostatic effect of lactoferrin on a serum susceptible and serum resistant strain of E. coli. Immunology. 1975 Jan;28(1):83–95. [PMC free article] [PubMed] [Google Scholar]
- Rocque W. J., Coughlin R. T., McGroarty E. J. Lipopolysaccharide tightly bound to porin monomers and trimers from Escherichia coli K-12. J Bacteriol. 1987 Sep;169(9):4003–4010. doi: 10.1128/jb.169.9.4003-4010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Cheron A., Montreuil J., Dolby J. M. Bacteriostasis of a milk-sensitive strain of Escherichia coli by immunoglobulins and iron-binding proteins in association. Immunology. 1978 Oct;35(4):663–671. [PMC free article] [PubMed] [Google Scholar]
- Stephens S., Dolby J. M., Montreuil J., Spik G. Differences in inhibition of the growth of commensal and enteropathogenic strains of Escherichia coli by lactotransferrin and secretory immunoglobulin A isolated from human milk. Immunology. 1980 Nov;41(3):597–603. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., de Graaff P., Tommassen J. Shielding of Escherichia coli outer membrane proteins as receptors for bacteriophages and colicins by O-antigenic chains of lipopolysaccharide. J Bacteriol. 1986 Oct;168(1):449–451. doi: 10.1128/jb.168.1.449-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]