Abstract
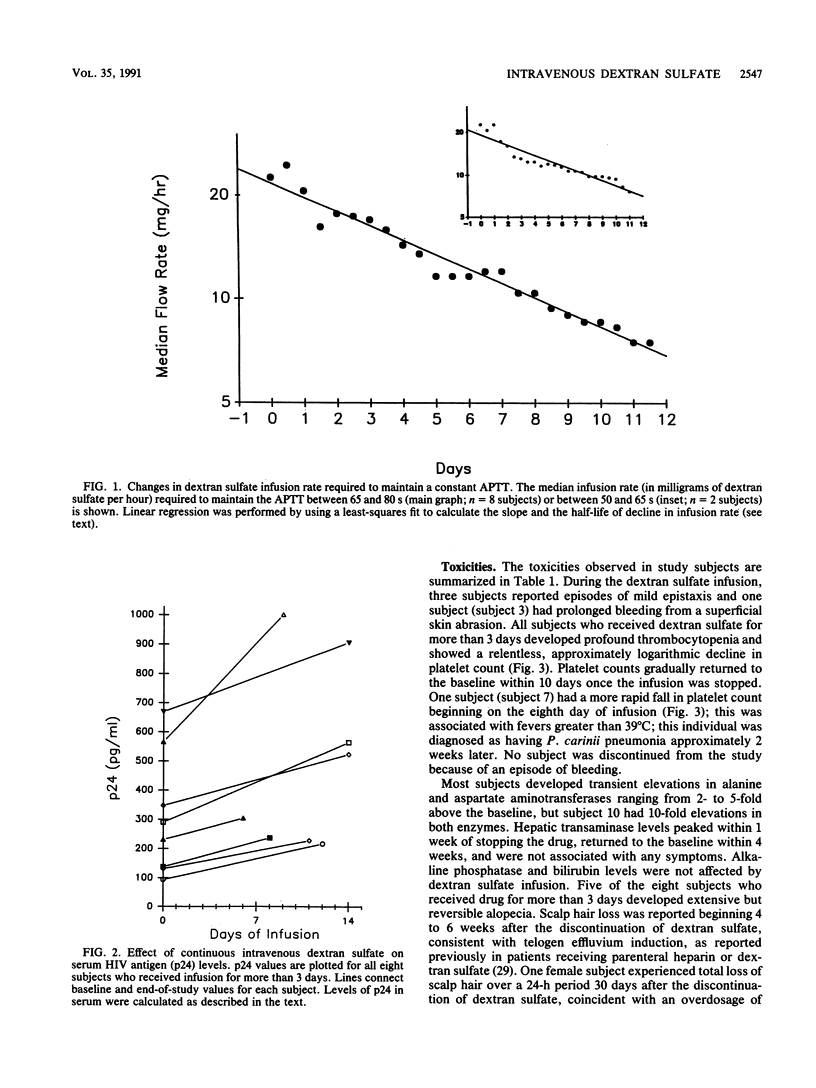
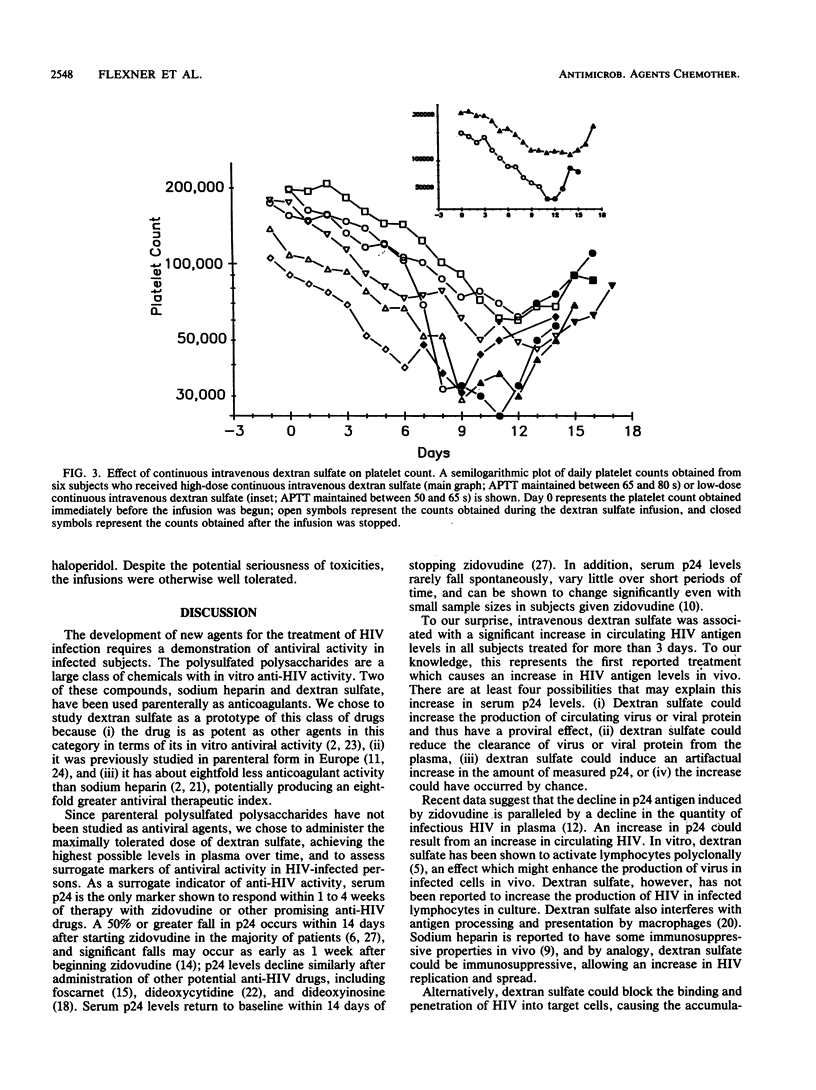
Polysulfated polysaccharides are attractive candidates for antiviral drug development because of their potent in vitro activities against human immunodeficiency virus (HIV), herpesviruses, and other enveloped viruses. To determine the potential anti-HIV activity of a prototypical polysulfated polysaccharide, we administered the maximally tolerated dose of dextran sulfate by continuous intravenous infusion to 10 subjects with symptomatic HIV infection for up to 14 days. Since parenteral dextran sulfate is an anticoagulant, the infusion was adjusted to produce the greatest acceptable increase in activated partial thromboplastin time. Drug concentrations in plasma achieved with this protocol were up to 200-fold greater than the 50% inhibitory concentration for free HIV infectivity in vitro. Despite this, circulating HIV antigen (p24) levels increased in all eight subjects who received the drug for more than 3 days (median proportional increase, 73.5%; range, 32 to 130%); this increase was highly significant when it was compared with that in a large cohort of untreated historical controls (Fisher's exact test, P less than 0.001). Frequent decreases in infusion rate were required in all subjects to maintain a constant activated partial thromboplastin time; plasma dextran sulfate levels did not fall as the infusion rate decreased, suggesting a decline in estimated drug clearance over time. Continuous intravenous dextran sulfate was toxic, producing profound but reversible thrombocytopenia in all eight subjects who received drug for more than 3 days and extensive but reversible alopecia in five of these subjects. Because of its toxicity and lack of beneficial effect on surrogate markers, dextran sulfate is unlikely to have a practical role in the treatment of symptomatic HIV infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams D. I., Kuno S., Wong R., Jeffords K., Nash M., Molaghan J. B., Gorter R., Ueno R. Oral dextran sulfate (UA001) in the treatment of the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. Ann Intern Med. 1989 Feb 1;110(3):183–188. doi: 10.7326/0003-4819-110-3-183. [DOI] [PubMed] [Google Scholar]
- Baba M., Nakajima M., Schols D., Pauwels R., Balzarini J., De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antiviral Res. 1988 Sep;9(6):335–343. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- Baba M., Pauwels R., Balzarini J., Arnout J., Desmyter J., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Snoeck R., Pauwels R., de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988 Nov;32(11):1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson R. E., Leuther M. D., Allain J. P., Nusinoff-Lehrman S., Boone G. S., Feigal D., Volberding P. Effect of zidovudine on serum human immunodeficiency virus core antigen levels. Results from a placebo-controlled trial. Arch Intern Med. 1988 Oct;148(10):2151–2153. [PubMed] [Google Scholar]
- Górski A., Wasik M., Nowaczyk M., Korczak-Kowalska G. Immunomodulating activity of heparin. FASEB J. 1991 Jun;5(9):2287–2291. doi: 10.1096/fasebj.5.9.1860620. [DOI] [PubMed] [Google Scholar]
- Hendrix C. W., Volberding P. A., Chaisson R. E. HIV antigen variability in ARC/AIDS. J Acquir Immune Defic Syndr. 1991;4(9):847–850. [PubMed] [Google Scholar]
- Ho D. D., Moudgil T., Alam M. Quantitation of human immunodeficiency virus type 1 in the blood of infected persons. N Engl J Med. 1989 Dec 14;321(24):1621–1625. doi: 10.1056/NEJM198912143212401. [DOI] [PubMed] [Google Scholar]
- Ito M., Baba M., Sato A., Pauwels R., De Clercq E., Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Res. 1987 Jul;7(6):361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Jackson G. G., Paul D. A., Falk L. A., Rubenis M., Despotes J. C., Mack D., Knigge M., Emeson E. E. Human immunodeficiency virus (HIV) antigenemia (p24) in the acquired immunodeficiency syndrome (AIDS) and the effect of treatment with zidovudine (AZT). Ann Intern Med. 1988 Feb;108(2):175–180. doi: 10.7326/0003-4819-108-2-175. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Crowe S., Levy J., Aweeka F., Gambertoglio J., McManus N., Mills J. Effect of Foscarnet therapy on infection with human immunodeficiency virus in patients with AIDS. J Infect Dis. 1988 Oct;158(4):862–865. [PubMed] [Google Scholar]
- Kahn J. O., Allan J. D., Hodges T. L., Kaplan L. D., Arri C. J., Fitch H. F., Izu A. E., Mordenti J., Sherwin J. E., Groopman J. E. The safety and pharmacokinetics of recombinant soluble CD4 (rCD4) in subjects with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase 1 study. Ann Intern Med. 1990 Feb 15;112(4):254–261. doi: 10.7326/0003-4819-112-4-. [DOI] [PubMed] [Google Scholar]
- Karpas A., Hill F., Youle M., Cullen V., Gray J., Byron N., Hayhoe F., Tenant-Flowers M., Howard L., Gilgen D. Effects of passive immunization in patients with the acquired immunodeficiency syndrome-related complex and acquired immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9234–9237. doi: 10.1073/pnas.85.23.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. S., Seidlin M., Reichman R. C., Plank C. S., Laverty M., Morse G. D., Knupp C., McLaren C., Pettinelli C., Valentine F. T. 2',3'-dideoxyinosine (ddI) in patients with the acquired immunodeficiency syndrome or AIDS-related complex. A phase I trial. N Engl J Med. 1990 May 10;322(19):1333–1340. doi: 10.1056/NEJM199005103221901. [DOI] [PubMed] [Google Scholar]
- Levantine A., Almeyda J. Drug reactions. 23. Drug induced alopecia. Br J Dermatol. 1973 Nov;89(5):549–553. doi: 10.1111/j.1365-2133.1973.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Leyva-Cobian F., Unanue E. R. Intracellular interference with antigen presentation. J Immunol. 1988 Sep 1;141(5):1445–1450. [PubMed] [Google Scholar]
- Lorentsen K. J., Hendrix C. W., Collins J. M., Kornhauser D. M., Petty B. G., Klecker R. W., Flexner C., Eckel R. H., Lietman P. S. Dextran sulfate is poorly absorbed after oral administration. Ann Intern Med. 1989 Oct 1;111(7):561–566. doi: 10.7326/0003-4819-111-7-561. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Skowron G., Bozzette S. A., Richman D., Uttamchandani R., Fischl M., Schooley R., Hirsch M., Soo W., Pettinelli C. Circulating p24 antigen levels and responses to dideoxycytidine in human immunodeficiency virus (HIV) infections. A phase I and II study. Ann Intern Med. 1989 Feb 1;110(3):189–194. doi: 10.7326/0003-4819-110-3-189. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Looney D. J., Kuno S., Ueno R., Wong-Staal F., Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988 Apr 29;240(4852):646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- RICKETTS C. R., WALTON K. W., VAN LEUVEN B. D., BIRBECK A., BROWN A., KENNEDY A. C., BURT C. C. Therapeutic trial of the synthetic heparin analogue dextran sulphate. Lancet. 1953 Nov 14;265(6794):1004–1010. doi: 10.1016/s0140-6736(53)91306-3. [DOI] [PubMed] [Google Scholar]
- Schols D., Baba M., Pauwels R., De Clercq E. Flow cytometric method to demonstrate whether anti-HIV-1 agents inhibit virion binding to T4+ cells. J Acquir Immune Defic Syndr. 1989;2(1):10–15. [PubMed] [Google Scholar]
- Schooley R. T., Merigan T. C., Gaut P., Hirsch M. S., Holodniy M., Flynn T., Liu S., Byington R. E., Henochowicz S., Gubish E. Recombinant soluble CD4 therapy in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase I-II escalating dosage trial. Ann Intern Med. 1990 Feb 15;112(4):247–253. doi: 10.7326/0003-4819-112-4-247. [DOI] [PubMed] [Google Scholar]
- Spear J. B., Benson C. A., Pottage J. C., Jr, Paul D. A., Landay A. L., Kessler H. A. Rapid rebound of serum human immunodeficiency virus antigen after discontinuing zidovudine therapy. J Infect Dis. 1988 Nov;158(5):1132–1133. doi: 10.1093/infdis/158.5.1132. [DOI] [PubMed] [Google Scholar]
- TUDHOPE G. R., COHEN H., MEIKLE R. W. Alopecia following treatment with dextran sulphate and other anticoagulant drugs. Br Med J. 1958 May 3;1(5078):1034–1037. doi: 10.1136/bmj.1.5078.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele B., Braig H. R., Ehm I., Kunze R., Ruf B. Influence of sulfated carbohydrates on the accessibility of CD4 and other CD molecules on the cell surface and implications for human immunodeficiency virus infection. Eur J Immunol. 1989 Jun;19(6):1161–1164. doi: 10.1002/eji.1830190630. [DOI] [PubMed] [Google Scholar]
- Ueno R., Kuno S. Dextran sulphate, a potent anti-HIV agent in vitro having synergism with zidovudine. Lancet. 1987 Jun 13;1(8546):1379–1379. doi: 10.1016/s0140-6736(87)90681-7. [DOI] [PubMed] [Google Scholar]
- de Swart C. A., Nijmeyer B., Roelofs J. M., Sixma J. J. Kinetics of intravenously administered heparin in normal humans. Blood. 1982 Dec;60(6):1251–1258. [PubMed] [Google Scholar]


