Abstract
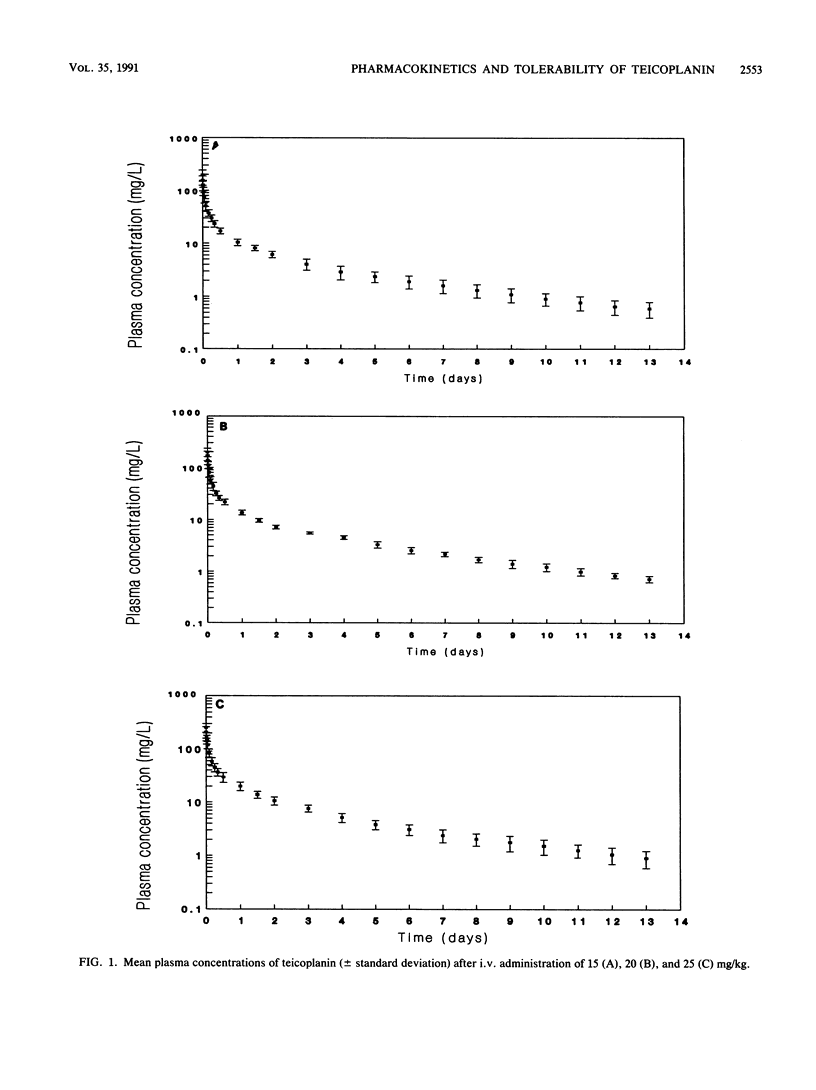
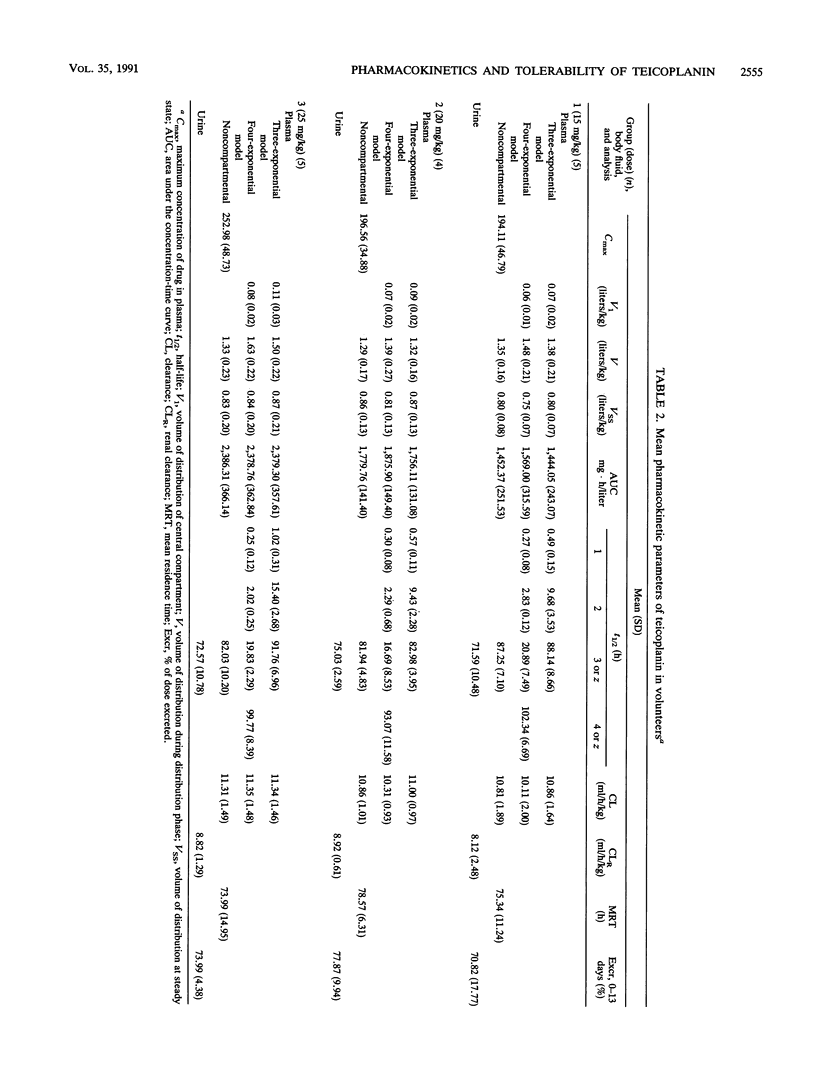
In this double-blind, randomized study, five healthy subjects per group received doses of 15, 20, or 25 mg of teicoplanin per kg of body weight, and one subject per group received a 0.9% NaCl placebo as single intravenous infusion over 30 min. Serial blood samples and urine were collected for 13 days postadministration, and concentrations of teicoplanin were determined by microbiological assay. The pharmacokinetic data were analyzed by noncompartmental and compartmental analyses. Laboratory safety tests, audiometry, and serum creatinine clearance measurements were done prior to day 1 and on days 2 and 14. In the three groups, peak levels at the end of the infusion averaged 194, 197, and 253 mg/liter, respectively. Mean concentrations in plasma 24 h after the administration were 10.5, 13.6, and 19.8 mg/liter, respectively. Mean values of volume of distribution at steady state were 0.80, 0.87, and 0.87 liters/kg, respectively. Terminal half-lives averaged 88, 83, and 92 h. Mean total clearance values were 10.9, 11.0, and 11.3 mg/h/kg, respectively, with renal clearance accounting for 75, 81, and 78%, respectively, of the total. The 13-day cumulative mean urinary recovery ranged from 71 to 78% of the dose within the groups. The pharmacokinetics of teicoplanin appears to be linear in the range of administered doses. Teicoplanin was generally well tolerated. Side effects, appearing in five subjects, were represented by fevers, chills, and skin reactions; these adverse reactions were mild, but one episode of rash necessitated the interruption of infusion, and one episode of chills necessitated treatment with corticosteroids. There was no indication of drug-related modifications of laboratory test results.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Buniva G., Del Favero A., Bernareggi A., Patoia L., Palumbo R. Pharmacokinetics of 14C-teicoplanin in healthy volunteers. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):23–28. doi: 10.1093/jac/21.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- Carver P. L., Nightingale C. H., Quintiliani R., Sweeney K., Stevens R. C., Maderazo E. Pharmacokinetics of single- and multiple-dose teicoplanin in healthy volunteers. Antimicrob Agents Chemother. 1989 Jan;33(1):82–86. doi: 10.1128/aac.33.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenaghi L., Corti A., Cassani G. Comparison of the solid phase enzyme receptor assay (SPERA) and the microbiological assay for teicoplanin. J Hosp Infect. 1986 Mar;7 (Suppl A):85–89. doi: 10.1016/0195-6701(86)90012-5. [DOI] [PubMed] [Google Scholar]
- Dalrymple P. D., Nicholls P. J. Tissue distribution and elimination of 14C-aminoglutethimide in the mouse. Eur J Drug Metab Pharmacokinet. 1990 Jan-Mar;15(1):31–35. doi: 10.1007/BF03190125. [DOI] [PubMed] [Google Scholar]
- Lam Y. W., Kapusnik-Uner J. E., Sachdeva M., Hackbarth C., Gambertoglio J. G., Sande M. A. The pharmacokinetics of teicoplanin in varying degrees of renal function. Clin Pharmacol Ther. 1990 May;47(5):655–661. doi: 10.1038/clpt.1990.87. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Reyes M. P., Cone L. A., Blair D. C., Jansen W., Wright G. E., Lorber R. R. Randomised, controlled trial of the comparative efficacy, auditory toxicity, and nephrotoxicity of tobramycin and netilmicin. Lancet. 1983 May 21;1(8334):1123–1126. doi: 10.1016/s0140-6736(83)92864-7. [DOI] [PubMed] [Google Scholar]
- Lewis P., Garaud J. J., Parenti F. A multicentre open clinical trial of teicoplanin in infections caused by gram-positive bacteria. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):61–67. doi: 10.1093/jac/21.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- Ripa S., Ferrante L., Mignini F., Falcioni E. Pharmacokinetics of teicoplanin. Chemotherapy. 1988;34(3):178–184. doi: 10.1159/000238568. [DOI] [PubMed] [Google Scholar]
- Rowland M. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 1990 Mar;18(3):184–209. doi: 10.2165/00003088-199018030-00002. [DOI] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner D. L. NONLIN84/PCNONLIN: software for the statistical analysis of nonlinear models. Methods Find Exp Clin Pharmacol. 1986 Oct;8(10):625–628. [PubMed] [Google Scholar]
- Wise R., Donovan I. A., McNulty C. A., Waldron R., Andrews J. M. Teicoplanin, its pharmacokinetics, blister and peritoneal fluid penetration. J Hosp Infect. 1986 Mar;7 (Suppl A):47–55. doi: 10.1016/0195-6701(86)90007-1. [DOI] [PubMed] [Google Scholar]
- Woo J., Floyd M., Cannon D. C. Albumin and beta 2-microglobulin radioimmunoassays applied to monitoring of renal-allograft function and in differentiating glomerular and tubular diseases. Clin Chem. 1981 May;27(5):709–713. [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]


