Abstract
Galactosialidosis (GS) is a human neurodegenerative disease caused by a deficiency of lysosomal protective protein/cathepsin A (PPCA). The GS mouse model resembles the severe human condition, resulting in nephropathy, ataxia, and premature death. To rescue the disease phenotype, GS mice were transplanted with bone marrow from transgenic mice overexpressing human PPCA specifically in monocytes/macrophages under the control of the colony stimulating factor-1 receptor promoter. Transgenic macrophages infiltrated and resided in all organs and expressed PPCA at high levels. Correction occurred in hematopoietic tissues and nonhematopoietic organs, including the central nervous system. PPCA-expressing perivascular and leptomeningeal macrophages were detected throughout the brain of recipient mice, although some neuronal cells, such as Purkinje cells, continued to show storage and died. GS mice crossed into the transgenic background reflected the outcome of bone marrow-transplanted mice, but the course of neuronal degeneration was delayed in this model. These studies present definite evidence that macrophages alone can provide a source of corrective enzyme for visceral organs and may be beneficial for neuronal correction if expression levels are sufficient.
Lysosomal storage diseases are caused by a deficiency of hydrolases that are essential for the correct degradative function of lysosomes (1, 2). Patients with these diseases develop systemic organ pathology and neurodegeneration because of the progressive lysosomal accumulation of toxic metabolites in various tissues, including the brain. Therapeutic strategies have relied on the unique capacity of soluble enzyme precursors to be secreted by one cell type and internalized via receptor-mediated endocytosis, by other cells at distant sites. Methods such as enzyme replacement therapy, bone marrow transplantation (BMT), organoid implantation, and gene therapy have been attempted in patients and animal models (reviewed in refs. 3 and 4). Each approach presents inherent problems mainly related to the difficulty of correcting the central nervous system (CNS) pathology. BMT, which relies on available donors, has been attempted for treatment of patients with variable results (2, 5). In animal models, this procedure has proved efficacious in the amelioration of CNS pathology in some cases [e.g., canine mucopolysaccharidosis (MPS) I and feline α-mannosidosis] (6, 7), but offers little or no benefit in others (e.g., murine MPS VII, canine GM1-gangliosidosis, and feline GM2-gangliosidosis) (8–11). Other approaches, including ex vivo gene therapy, have suffered from poor transduction efficiencies, short-term or silenced gene expression in vivo, and the difficulty of delivering therapeutic protein to target cells (12, 13).
Our strategy, which overcomes many of these obstacles, is to generate transgenic mice that express the therapeutic protein at sustained levels in a specific BM cell lineage and to transplant their BM into deficient mice. The disease model used in these studies is galactosialidosis (GS) (reviewed in ref. 14), which is caused by a primary deficiency of protective protein/cathepsin A (PPCA). PPCA has carboxypeptidase/deamidase activity, forms a complex with lysosomal neuraminidase and β-galactosidase, and, when absent, leads to a secondary deficiency of both hydrolases. The GS mouse model closely mimics the human disease (15), developing extensive vacuolation of specific cells in most organs and oligosacchariduria. Transplantation of GS mice with BM from transgenic mice overexpressing human PPCA in the erythroid cell lineage resulted in complete correction of GS visceral pathology, but only minor amelioration of the brain disease. The latter was likely the result of expression/secretion of endogenous mouse PPCA by BM-derived macrophages that had infiltrated the brain (15).
Here, we have investigated whether BM-derived macrophages and microglia overexpressing the corrective protein might afford better correction of organs, including the CNS. The human colony-stimulating factor-1 receptor (CSF-1R) promoter (16) was used to drive expression of a human PPCA minigene specifically in macrophages of transgenic mice. We demonstrate that transgenic BM, transplanted into GS mice, is remarkably effective in ameliorating the disease process.
MATERIALS AND METHODS
Construction of the CSF-1R/Human PPCA Transgene.
The human PPCA cDNA (17) was ligated to the rabbit β-globin splice site and polyadenylation signal and cloned into pIC20H (18). To enhance translation efficiency, the PPCA translation initiation sequence was replaced with that of the rabbit β-globin gene, thereby adding a HindIII site at the 5′ end of the cDNA. A 3.35-kb PPCA minigene was generated by replacing an internal 0.3-kb BamHI cDNA fragment with the corresponding genomic fragment, including introns 4–6. A 5.3-kb portion of the human CSF-1R promoter was obtained by cutting a genomic CSF-1R clone (kind gift of Thomas Look, St. Jude Children’s Research Hospital, Memphis, TN) at the translation initiation site with NcoI and removing the ATG with S1 nuclease before digestion with SpeI. The PPCA minigene was inserted downstream of the CSF-1R promoter (Fig. 1A). The fragment (8.65-kb) used for DNA injections was excised with NotI/SalI.
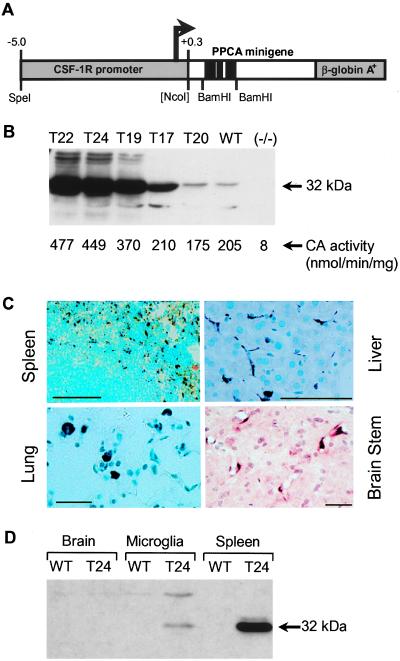
Figure 1.
Structure and expression of a CSF-1R-PPCA transgene in transgenic mice. (A) Schematic representation of the expression vector. (B) BM lysates were analyzed on a Western blot probed with α32 antibodies. (B, Bottom) Cathepsin A activity was measured in BM lysates from two mice of each transgenic line (age 2–6 months). These values are indicative of those obtained in three independent experiments. (C) Immunocytochemistry of transgenic mouse tissues. Transgenic mouse (T22) sections of spleen, liver, lung, and brain stem were probed with α32 antibodies. (D) Western blot comparing human PPCA expression in 20 μg of whole brain, a microglia-enriched cell population, and spleen from wild-type and transgenic (T24) mice. This blot is indicative of results obtained in three separate experiments.
Generation of Transgenic Mice and Genotype Analysis.
Transgenic mice were generated by using the FVB/NJ strain (19) and were identified by tail blots of HindIII-digested DNA probed with the 0.9-kb BamHI fragment of the human PPCA gene. The copy number of the transgene in each founder was determined by comparison with defined amounts of the human PPCA minigene.
Enzyme Assays and Western Blot Analysis.
Tissue lysates were assayed for cathepsin A, neuraminidase, and β-galactosidase activities (20). The same lysates also were run on 12.5% SDS-polyacrylamide gels (SDS/PAGE), under reducing conditions, and blotted onto Hybond-P poly(vinylidene difluoride) membranes (Amersham). Antibodies against the 32-kDa subunit of human PPCA (α32) were raised in rabbits (20) and affinity-purified against the human protein. Western blots were incubated overnight with purified α32 antibodies followed by a 2-hr incubation with horseradish peroxidase-conjugated, goat anti-rabbit secondary antibodies (Sigma). Binding was visualized by using a chemiluminescence substrate (NEN). The α32 antibodies have a high affinity for the human protein and crossreact poorly with mouse PPCA. Moreover, the human 32-kDa subunit on SDS/PAGE has a slightly lower molecular mass than its mouse counterpart.
Immunocytochemical and Histochemical Staining of Mouse Tissues.
Mice were perfused with 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4. Dissected organs were processed for paraffin embedding. Tissue sections were deparaffinized, rehydrated, and antigen-retrieved by microwave boiling in 0.1 M citrate, pH 6.0. The sections were blocked for 30 min and incubated overnight with either α32 or PEP-19 antibodies. Detection was performed with the ABC horseradish peroxidase system using a VIP (purple) or diaminobenzidine (brown) substrate (Vector). For Mac-1 antibody (rat anti-mouse CD11b-PharMingen) staining, fixed tissues were placed in a solution of 30% sucrose/0.1 M sodium phosphate, pH 7.4 for 24–48 hr at 4°C before embedding in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC). Cryostat sections were probed with antibodies as above. Paraffin sections were stained with periodic acid/Schiff or hematoxylin/eosin (H&E) by using standard methods.
Isolation of Microglia.
Microglia were isolated from the brains of 7- to 10-day-old pups by fractionation on a discontinuous Percoll gradient (21). The enriched microglia fraction (90–95% pure) was washed in PBS and frozen as a pellet.
BMT.
Recipient GS mice (C57BL/6×129/J×FVB/NJ) of ages 1, 2.5, and 7–9 months were lethally irradiated with 830 rad 24 hr before transplantation. One-3 homozygous CSF-1R–human PPCA transgenic mice served as BM donors while normal BM was obtained from wild-type FVB/NJ mice. BM cells were incubated on ice for 30 min with an anti-mouse CD3 antibody made in guinea pig (H57–597) (kind gift of Peter Doherty, St. Jude Children’s Research Hospital). After being washed, the cells were subjected to complement lysis (five parts Low-Tox guinea pig complement/one part rabbit complement, Cedarlane Laboratories) at 37°C for 45 min to achieve T cell depletion. Five-hundred microliters of a cell suspension containing 4 × 107 cells/ml was injected via the tail vein. Treated mice were analyzed at various times (2–12 months) posttransplantation.
Analysis of Urinary Oligosaccharides.
Urine samples were tested for the presence of undegraded oligosaccharides by using a FACE urinary carbohydrate analysis kit (Glyko, Novato, CA).
Coordination Testing on a Rota-Rod.
Mice were trained twice on 2 consecutive days for a 5-min period at low speed (3 rpm) to become accustomed to the accelerating rota-rod treadmill (Stoelting). On the third and fourth days, they were placed on the rota-rod at accelerating speeds from 3 to 30 rpm (increments of 3 rpm/30 sec) and tested twice with a 5-min break between tests. The mice were kept on the apparatus for a maximum of 280 sec. For each mouse, a single measurement was calculated to represent the average performance of these four attempts. All testing was performed between 2 and 5 p.m.
RESULTS
CSF-1R Drives Human PPCA Expression Specifically in Macrophage-Derived Cells.
To generate transgenic mice, we designed a construct with 5.3 kb of the human CSF-1R promoter located upstream of a human PPCA minigene (Fig. 1A). Five independent transgenic founders were obtained with single integration sites for the transgene, ranging in copy number from 5 to 35 (i.e., T20, T17, T19, T24, and T22 had 5, 12, 15, 25, and 35 copies, respectively). They were bred to homozygosity before analysis. Human PPCA expression was assessed by measuring cathepsin A activity in the founder’s BM. The three transgenic lines with the highest copy numbers gave activities 1.8- to 2.4-fold higher than controls (Fig. 1B, Lower). Similar values were measured in the spleen, whereas in other tissues, including liver, kidney, lung, and brain, there was little or no detectable increase in activity over endogenous levels (not shown). Consistent with these results, Western blots of transgenic BM (Fig. 1B) and spleen demonstrated high human PPCA expression that correlated with the transgene copy number. Lower, but significant, expression was seen in lung, liver, kidney, and brain, but not in testis (not shown). This variation of expression in different tissues could reflect differences in CSF-1R promoter regulation at sites where macrophage colonization/infiltration is required (22) and/or different demands for tissue macrophages in the animals at the time of sacrifice.
The distribution of the transgenic protein in the target cells was analyzed in tissue sections probed for human PPCA (Fig. 1C). Both in hematopoietic and nonhematopoietic tissues, human PPCA expression was confined solely to lysosomes (i.e., punctate staining pattern) of macrophages and macrophage-derived cells. When we used staining intensity as a measure of PPCA expression, alveolar macrophages (Fig. 1C, Lung) consistently expressed at higher levels than splenic macrophages (Fig. 1C, Spleen), which in turn expressed higher than Kupffer cells (Fig. 1C, Liver). Hence, the microenvironment of macrophages specifies the level of PPCA expression under the CSF-1R promoter.
In brain, moderately stained perivascular macrophages/microglia were found throughout the parenchyma, including the olfactory bulb, cerebrum, cerebellum, and brain stem (Fig. 1C, Brain Stem). Outside the blood-brain barrier, scattered positive macrophages were detected in the choroid plexus and leptomeningeal macrophages (not shown). Human PPCA, however, could not be visualized in ramified microglia, either because it was confined to the lysosomes of the very fine processes of these cells or because the level of expression was too low. To verify transgene expression, a purified preparation of microglia from wild-type and transgenic (T24) mice was analyzed by Western blotting (Fig. 1D). Human PPCA levels were clearly higher in the microglia-enriched transgenic sample compared with the total brain lysate, although expression was low compared with that in the spleen. Similar results were obtained with transgenic line T22 (not shown). Together these data demonstrate that the human CSF-1R promoter functions in the same tissue-specific manner in visceral tissues and brain as the endogenous gene from which it is derived (23–26).
Transplantation of GS Mice with Transgenic BM Corrects the Disease Phenotype.
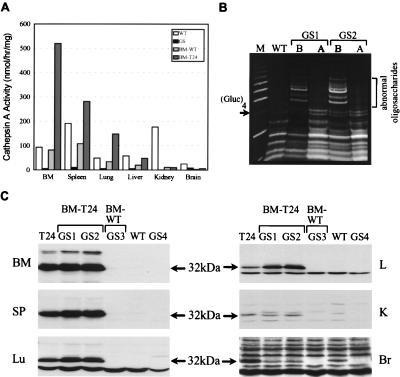
Affected mice were transplanted at 1 month of age with BM from either transgenic or wild-type mice and analyzed 2–12 months post-BMT. Treated mice appeared normal despite their smaller size, which is common for mice irradiated at an early age. Although GS mice developed ataxia at 5–6 months of age, no signs of ataxia were evident in the transplanted mice even 1 year after BMT. Oligosacchariduria was completely reverted by BMT (Fig. 2B) already after 1 month, and this correction persisted for the life of the animals. Mice with transgenic BM maintained levels of cathepsin A activity equal to or higher than wild-type mice in their BM, spleen, lung, and liver (Fig. 2A). No significant increase was seen in kidney and brain. However, considering that the distribution of endogenous PPCA in the latter tissues of wild-type mice is confined primarily to nonmacrophage-derived cells (27), restoration of enzyme activity in BMT mice might not be measurable even after efficient engraftment. Further, microglia turnover is slow and BM-derived transgenic macrophages may not yet have efficiently repopulated (28–30). Concomitant with restoration of cathepsin A activity was a proportionate increase in neuraminidase activity (not shown). We also noticed that, with the exception of cultured fibroblasts, tissues from PPCA(−/−) mice display a gradual increase in β-galactosidase activity during disease progression, and this activity is normalized upon engraftment after BMT (unpublished data). The mechanism underlying this phenomenon is still unclear.
Figure 2.
Urinary oligosaccharides and human PPCA expression in GS mice after BMT. (A) Cathepsin A activities in tissues of BMT mice. GS mice, transplanted with either wild-type or transgenic (T24) BM at 2.5 months of age were sacrificed 2 months later, and tissue lysates were assayed for cathepsin A activity. (B) Urine samples were collected from 1-month-old wild-type and GS mice before BMT (lanes B) and 1 month after BMT (lanes A). Mouse GS1 and GS2 received wild-type and transgenic BM, respectively. A molecular weight ladder of glucose polymers (M), with the (Glucose)4 for reference, is shown. Oligosaccharides larger than this tetrasaccharide are abnormal. (C) Western blot of tissues from BMT mice were probed with α32 antibodies: GS1 and GS2 mice received transgenic BM (BM-T24) and GS3 mouse received wild-type BM (BM-WT). The exposure time for the brain was 12 times longer than that for the other tissues.
Western blotting showed human PPCA only in those mice transplanted with transgenic BM (Fig. 2C). In spleen, lung, and liver, expression was consistently higher in BMT mice than in donor transgenic mice. This increase probably was caused by the extra macrophage recruitment of tissues to remove cellular debris from dead and dying cells. Interestingly, human PPCA was detected in the brain, but at a significantly lower level than for the transgenic animals, probably because of the slow turnover rate of microglia in BMT mice. These results clearly demonstrate that PPCA-expressing cells can infiltrate virtually any tissue.
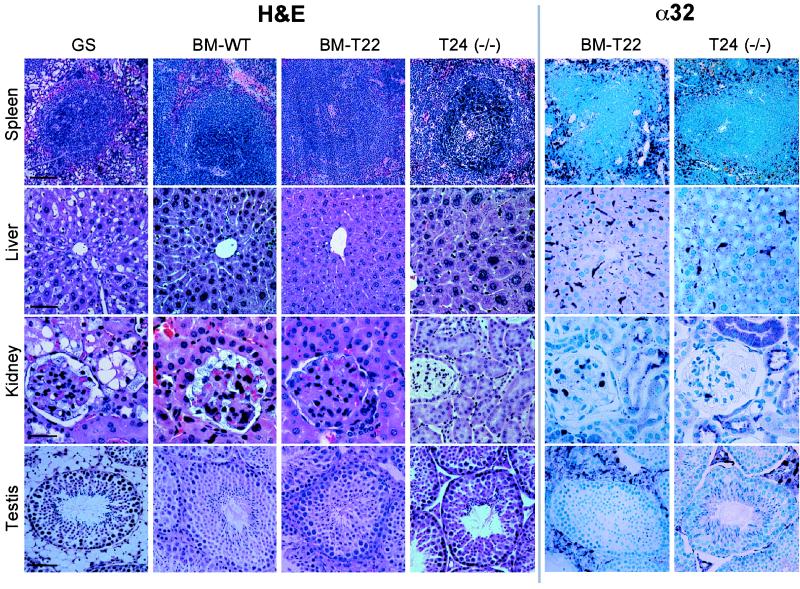
To ascertain the improvement of tissue morphology after BMT, H&E staining was performed (Fig. 3, H&E). The extensive vacuolation observed in visceral organs of untreated GS mice (spleen, liver, kidney, and testis are shown as examples) was largely and similarly corrected by wild-type (BM-WT) and transgenic BM. However, in the kidney, transgenic BM (BM-T22) clearly afforded better correction than BM-WT, particularly in glomerular visceral epithelial cells that are known to require more therapeutic protein for correction (15), indicating that macrophage-derived human PPCA was secreted, effectively taken up, and processed into its active form. Interestingly, although the interstitial cells of the caput epididymis were corrected, the tubular cells continued to store even in mice receiving transgenic BM (not shown). Immunocytochemistry confirmed the presence of human PPCA-expressing macrophages in every tissue tested (Fig. 3, α32 and BM-T22). This presence was most obvious in BM, spleen, lung, and liver. Notably, in the Bowman’s capsule and proximal convoluted tubules of the kidney (Fig. 3, α32 and BM-T22) and in hepatocytes (Fig. 3, α32 and BM-T22), internalized protein accumulated in lysosomes to such an extent that it could be detected as punctate staining. In the testis, many PPCA-expressing macrophages were seen among the Leydig cells (Fig. 3, α32 and BM-T22), whereas relatively few infiltrated into the caput epididymis (not shown). This observation explains the complete correction of the testis compared with the epididymis. In general, visceral organ correction was impressive using this system, even if GS mice were transplanted late in life (7–9 months). These results could be relevant for future human applications. The transplanted mice displayed no obvious signs of an immune response to either introduce murine or human PPCA.
Figure 3.
Histology and immunocytochemistry of BMT and transgenic-knockout mice. GS mice (1 month old) were transplanted either with normal (BM-WT) or transgenic (BM-T22) BM and sacrificed 11.5 months later. Age-matched GS mice and transgenic-knockout [T24(−/−)] mice also were analyzed. Tissue sections were stained with H&E and labeled with α32 antibodies for immunocytochemistry. [Scale bar = 40 μm (kidney) or 80 μm (spleen, liver, testis).]
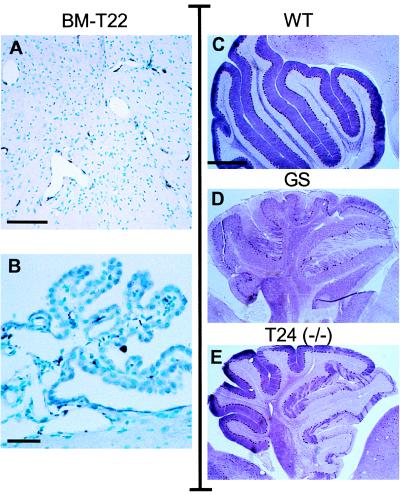
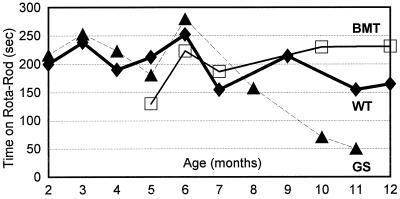
In the brain of transplanted mice, human PPCA expression was restricted to perivascular, leptomeningeal, and choroid plexus macrophages (Fig. 4 A and B). Although the choroid plexus was fully corrected, periodic acid/Schiff staining revealed that scattered neurons still accumulated undegraded products (not shown). Because of the regional distribution of accumulating cells in the CNS of GS mice (15), it is difficult to accurately estimate whether isolated neuronal cells have been cleared of storage. Therefore, we monitored the effect of BMT in the cerebellum that undergoes an obvious and dramatic loss of Purkinje cells in GS mice. The Purkinje cells in transplanted mice continued to store and die (2, 6, and 11.5 months post-BMT, not shown), indicating that the number of CNS macrophages/microglia and/or their level of secretion of corrective enzyme was insufficient to prevent their loss. More animals, sacrificed at different ages, will be required to determine whether a decreased rate of cell loss occurs after BMT considering that mice up to 11.5 months post-BMT performed at a level well above that of untreated GS mice when tested for motor coordination on a rota-rod (Fig. 5).
Figure 4.
Immunocytochemistry of the brain in BMT and transgenic-knockout mice. GS mice were transplanted with transgenic BM (BM-T22) and sacrificed 11.5 months later. Brain sections were stained with α32 antibodies and revealed expression in the perivascular macrophages of the brain stem (A) and macrophages of the choroid plexus (B). To visualize the Purkinje cells of the cerebellum, sections from 5-month-old (C), GS (D), and transgenic-knockout [T24(−/−)] (E) mice were stained with an anti-PEP-19 antibody. Note the marked loss of Purkinje cells in GS mice and the partial loss in T24(−/−) mice. [Scale bars = 60 μm (B), 120 μm (A), and 500 μm (C–E).]
Figure 5.
Coordination tests of GS and BMT mice on a rota-rod. Mice were placed on a rota-rod that accelerated from 3 to 30 rpm, and the time they remained on the apparatus was recorded. The number of mice tested at each month of age ranged from 1 to 14. Note, few GS mice survive beyond the age of 11–12 months.
Breeding of CSF-1R Human PPCA Mice into the PPCA −/− Background.
To assess the potential to correct this disease only by overexpressing macrophages, transgenic mice (T24) were cross-bred with GS mice to obtain transgenic knockout mice [T24(−/−)]. These mice developed no overt signs of disease and bred more prolifically than GS mice of a similar age. Analysis of their tissues by H&E staining [Fig. 3, H&E, T24(−/−)] and immunocytochemistry [Fig. 3, α32, T24(−/−)] revealed virtually identical results to those of mice receiving transgenic BM (BM-T22). The only difference was the better correction of glomerular cells in BMT mice, which may have resulted from these irradiated mice incurring damage, inducing an inflammatory response and hence acquiring a greater local concentration of PPCA-expressing cells. Alternatively, endogenous mouse PPCA in the transplanted transgenic BM may have contributed to the extra correction. In the cerebrum, cerebellum, and brain stem of T24(−/−) mice, PPCA-positive cells were present at perivascular and leptomeningeal sites (not shown). Again, we had difficulty detecting microglia by immunocytochemistry, and the results were virtually identical to those obtained for BMT mice. However, because these mice displayed no obvious disease symptoms and also performed better than GS mice on the rota-rod (not shown), we looked in detail at the morphology of their cerebella. Wild-type, GS, and T24(−/−) mice (Fig. 4 C–E) were analyzed by staining brain sections with an antibody against PEP-19, a protein specific for Purkinje cells in the cerebellum (31). In GS mice, Purkinje cell loss proceeded in an anterior to posterior direction during disease progression, similar to that reported for the Niemann-Pick mouse model (32). Cell death occurred in patches, although the cells of the inferior lobule were largely spared even in very old mice (up to 13.5 months) and was preceded by the accumulation of intralysosomal periodic acid/Schiff-positive granules (not shown). In T24(−/−) mice, the rate of Purkinje cell death was slower than that in GS mice, which was evidenced by the dramatic difference of PEP-19-positive cells surviving in 5-month-old T24(−/−) mice compared with age-matched knockouts (Fig. 4 D and E). This apparent improvement of cerebellar pathology could be the result of small amounts of human PPCA expressed by microglia or perivascular macrophages throughout the brain of these mice after birth and/or during their development.
DISCUSSION
For some time, macrophages have been viewed as a vehicle to overexpress and deliver therapeutic proteins to virtually all regions of the body, including the brain. This feature is particularly important for lysosomal storage disorders whose pathology involves both visceral organs and the CNS. In this study, we investigated the overall expression of a lysosomal marker/therapeutic gene (PPCA) under the control of a macrophage-specific promoter, CSF-1R, in transgenic mice. For such a therapeutic regime to be effective, it is imperative that the promoter remains active in the macrophages as they enter different cellular microenvironments. CSF-1R is expressed solely in monocyte/macrophage-derived cells (33), including the microglia of adult mouse, rat, and human brain (23–26). Its tissue specificity is determined, for the most part, at the transcriptional level (16, 34, 35); however, little is known about the level of expression obtained with this promoter in vivo in tissues other than BM or peripheral blood.
We overexpressed the PPCA in cells derived from the BM macrophage lineage. Expression varied in different tissues or even in the same tissue, probably reflecting cell-specific CSF-1R promoter regulation upon differentiation or in response to signals from the surrounding microenvironment. In some tissues, PPCA levels correlated with copy number. It is unclear whether the CSF-1R promoter fragment used contains a locus control region-type element, to enhance and insulate the gene it activates from surrounding influences. After BMT in GS mice, both wild-type and transgenic BM corrected much of the visceral pathology, although there was a clear indication that overexpressing cells contributed to better correction. More compelling evidence came from the transgenic knockout mice, where virtually all visceral organs were corrected to some degree. We noticed that the level of human PPCA in transgenic mouse serum or that secreted from cultured transgenic BM macrophages was quite low (unpublished data), indicating that therapeutic amounts of the corrective protein may vary for different cell types. Many lysosomal enzymes are present in excess, and it is possible that some cells have a higher threshold requirement of enzyme for correction than others, for example, in these studies, the glomerular visceral and caput epididymis epithelial cells. Cells with a low threshold include the renal proximal convoluted tubule cells and Leydig cells. Interestingly, the wild-type glomerulus displays very low levels of endogenous mouse PPCA, whereas the proximal convoluted tubule epithelium expresses moderate levels and the epididymis high levels (27). Therefore, endogenous protein expression is not an indication of the overall requirement of this protein for normal metabolic processes. Further, the level of substrate in some cells may change as a result of the disease or there may be other barriers in certain cell types that prevent PPCA uptake.
One year after transplantation with transgenic BM, numerous PPCA-positive perivascular and leptomeningeal macrophages were seen throughout the CNS. Similar results have been obtained by others (30, 36–38). However, affected neurons, such as Purkinje cells, continued to accumulate undegraded products and die. These cells are notoriously difficult to correct (39), although it was shown recently that BMT slowed their loss in Niemann-Pick disease mice (40). Transplanted GS mice, despite losing their Purkinje cells, performed better in motor coordination tests than their age-matched untreated siblings. This finding suggests that their ataxic phenotype and progressive lack of coordination is complex, involving not only neuronal but also significant visceral factors. In our transgenic knockout model, in which all perivascular macrophages and microglia expressed PPCA to some extent, Purkinje cell storage and death was delayed. This delay may be because of the presence of PPCA throughout development and implies that these neurons can take up the corrective protein. However, higher enzyme levels will be required to prevent cell death over an extended period. Alternatively, amelioration of this neuronal pathology may be secondary to the correction of peripheral organs, resulting in lower circulating levels of undegraded products.
GS mice, while fertile, mate poorly and consequently have few litters. Yet, transgenic knockout mice overexpressing the corrective protein specifically in erythroid (15) or macrophage-derived cells display normal pregnancy and delivery patterns (unpublished data). This apparent reduction in fertility is unlikely to be caused by neurologic disturbances in mating behavior, but rather visceral factors because virtually total amelioration of visceral pathology occurs. Further, correction of storage in Leydig cells, but not in epididymal cells, may contribute to this improvement.
We have shown that macrophages alone conclusively correct pathology of affected cells in vivo. It is noteworthy that wild-type macrophages, transplanted into mucopolysaccharidosis VII mice, seemed to partially ameliorate disease pathology, as reported in abstract form (41). Our results indicate that higher levels of expression afford a more timely and effective correction. It remains to be seen whether enough macrophages will ever repopulate the CNS to allow for neural correction even upon BMT at an early age. When considering the potential efficacy for cell therapy, issues to be taken into account include the level of expression/secretion of the therapeutic protein from donor cells, the number of donor cells either locally or in total, the capacity of target cells to take up the therapeutic protein, and the secondary correction of affected cells because of clearance of toxic products elsewhere. Furthermore, successful therapy for any given lysosomal disease may depend heavily on the properties of the individual enzyme, such as its stability in body fluids, whether it is membrane associated, and how efficiently it is secreted and reinternalized. Thus, the level of overexpression necessary to achieve a therapeutically beneficial response will be unique for each lysosomal storage disease being treated.
Acknowledgments
We are very grateful to Drs. Gerard Grosveld, William Walker, Carin Havenith, Peter Doherty, Tom Look, Jim Morgan, Richard Smeyne, and Susan Watson. We are indebted to Christy Nagy, John Swift, Charlette Hill, Erik Bonten, and Sjozef van Baal of St. Jude and Chris Starr of Glyko. This work was supported by the Assisi Foundation of Memphis, National Institutes of Health Grant DK-52025, the Cancer Center (CORE) Support Grant CA-21765, and the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital.
ABBREVIATIONS
- GS
galactosialidosis
- PPCA
protective protein/cathepsin A
- CSF-1R
colony-stimulating factor-1 receptor
- BM
bone marrow
- BMT
BM transplantation
- CNS
central nervous system
- H&E
hematoxylin/eosin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Neufeld E F. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- 2.Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. [Google Scholar]
- 3.Rattazzi M C, Dobrenis K. In: Treatment of Genetic Diseases. Desnick R J, editor. New York: Churchill Livingstone; 1991. pp. 131–152. [Google Scholar]
- 4.Haskins M, Baker H J, Birkenmeier E, Hoogerbrugge P, Poorthuis B, Sakiyama T, Shull R, Taylor R, Thrall M, Walkley S U. Treatment of Genetic Diseases. New York: Churchill Livingstone; 1991. [Google Scholar]
- 5.Hoogerbrugge P M, Brouwer O F, Bordigoni P, Ringden O, Kapaun P, Ortega J J, O’Meara A, Cornu G, Souillet G, Frappaz D, et al. Lancet. 1995;345:1398–1402. doi: 10.1016/s0140-6736(95)92597-x. [DOI] [PubMed] [Google Scholar]
- 6.Shull R M, Hastings N E, Selcer R R, Jones J B, Smith J R, Cullen W C, Constantopoulos G. J Clin Invest. 1987;79:435–443. doi: 10.1172/JCI112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walkley S U, Thrall M A, Dobrenis K, Huang M, March P A, Siegel D A, Wurzelmann S. Proc Natl Acad Sci USA. 1994;91:2970–2974. doi: 10.1073/pnas.91.8.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien J S, Storb R, Raff R F, Harding J, Appelbaum F, Morimoto S, Kishimoto Y, Graham T, Ahern-Rindell A, O’Brien S L. Clin Genet. 1990;38:274–280. doi: 10.1111/j.1399-0004.1990.tb03581.x. [DOI] [PubMed] [Google Scholar]
- 9.Birkenmeier E H, Barker J E, Vogler C A, Kyle J W, Sly W S, Gwynn B, Levy B, Pegors C. Blood. 1991;78:3081. [PubMed] [Google Scholar]
- 10.Wolfe J H, Deshmane S L, Fraser N W. Nat Genet. 1992;1:379–384. doi: 10.1038/ng0892-379. [DOI] [PubMed] [Google Scholar]
- 11.Walkley S, Thrall M, Dobrenis K, March P A, Siegel D A, Wurzelmann S. J Neuropathol Exp Neurol. 1993;52:315a. (abstr.). [Google Scholar]
- 12.Crystal R. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 13.Marshall E. Science. 1995;269:1050–1055. doi: 10.1126/science.7652552. [DOI] [PubMed] [Google Scholar]
- 14.d’Azzo A, Andria G, Strisciuglio P, Galjaard H. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C, Beaudet A, Sly W, Valle D, editors. Vol. 2. New York: McGraw-Hill; 1995. pp. 2825–2838. [Google Scholar]
- 15.Zhou X Y, Morreau H, Rottier R, Davis D, Bonten E, Gillemans N, Wenger D, Grosveld F G, Doherty P, Suzuki K, et al. Genes Dev. 1995;9:2623–2634. doi: 10.1101/gad.9.21.2623. [DOI] [PubMed] [Google Scholar]
- 16.Roberts W M, Shapiro L H, Ashmun R A, Look A T. Blood. 1992;79:586–593. [PubMed] [Google Scholar]
- 17.Galjart N J, Gillemans N, Harris A, van der Horst G T J, Verheijen F W, Galjaard H, d’Azzo A. Cell. 1988;54:755–764. doi: 10.1016/s0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- 18.Marsh J, Erfle M, Wykes E. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 19.Grosveld F, Blom van Assendelft G, Greaves D R, Kollias G. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 20.Galjart N J, Morreau H, Willemsen R, Gillemans N, Bonten E J, d’Azzo A. J Biol Chem. 1991;266:14754–14762. [PubMed] [Google Scholar]
- 21.Havenith C, Askew D, Walker W. GLIA. 1998;22:348–359. [PubMed] [Google Scholar]
- 22.Sapi E, Flick M, Rodov S, Gilmore-Hebert M, Kelley M, Rockwell S, Kacinski B. Cancer Res. 1996;56:5704–5712. [PubMed] [Google Scholar]
- 23.Raivich G, Gehrmann J, Kreutzberg G. J Neurosci. 1991;30:682–686. doi: 10.1002/jnr.490300412. [DOI] [PubMed] [Google Scholar]
- 24.Moore S C, McCormack J M, Armendariz E, Gatewood J, Walker W S. J Neuroimmunol. 1992;41:203–214. doi: 10.1016/0165-5728(92)90071-r. [DOI] [PubMed] [Google Scholar]
- 25.Chang Y, Albright S, Lee F. J Neuroimmunol. 1994;52:9–17. doi: 10.1016/0165-5728(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 26.Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer P L. Brain Res. 1994;639:171–174. doi: 10.1016/0006-8993(94)91779-5. [DOI] [PubMed] [Google Scholar]
- 27.Rottier R, Hahn C, Mann L, Martin M, Smeyne R, Suzuki K, d’Azzo A. Hum Mol Gen. 1998;7:1787–1794. doi: 10.1093/hmg/7.11.1787. [DOI] [PubMed] [Google Scholar]
- 28.Paabo S, Bhat B M, Wold W S, Peterson P A. Cell. 1987;50:311–317. doi: 10.1016/0092-8674(87)90226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry V H, Gordon S. In: International Review of Cytology: A Survey of Cell Biology. Jeon K W, Friedlander M, editors. Vol. 125. San Diego: Academic; 1991. pp. 203–244. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy D, Abkowitz J. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 31.Ziai M, Sangameswaran L, Hempstead J, Danho W, Morgan J. J Neurochem. 1988;51:1771–1776. doi: 10.1111/j.1471-4159.1988.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuemmel T, Schroeder R, Stoffel W. J Neuropathol Exp Neurol. 1997;56:171–179. doi: 10.1097/00005072-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Sherr C J, Rettenmier C W, Sacca R, Roussel M F, Look A T, Stanley E R. Cell. 1985;41:665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 34.Reddy M A, Yang B, Yue X, Barnett C J K, Ross I L, Sweet M J, Hume D A, Ostrowski M C. J Exp Med. 1994;180:2309–2319. doi: 10.1084/jem.180.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin D I, Jameson S B, Reddy M A, Schenkman D, Ostrowski M C. Mol Cell Biol. 1995;15:693–703. doi: 10.1128/mcb.15.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krall W J, Challita P M, Perlmutter L S, Skelton D C, Kohn D B. Blood. 1994;83:2737–2748. [PubMed] [Google Scholar]
- 37.Hickey W F, Kimura H. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 38.Hickey W F, Vass K, Lassmann H. J Neuropath Exp Neurol. 1992;51:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Sands M, Vogler C, Torrey A, Levy B, Gwynn B, Grubb J, Sly W, Birkenmeier E. J Clin Invest. 1997;99:1596–1605. doi: 10.1172/JCI119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miranda S, Erlich S, Freidrich V, Haskins M, Gatt S, Schuchman E. Transplantation. 1998;65:884–892. doi: 10.1097/00007890-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 41.Freeman B, Vogler C, Hofling A, Nicholes A, Sands M. Blood. 1997;90:522a. (abstr.). [PubMed] [Google Scholar]