Abstract
Sickle cell anemia (SCA) and thalassemia are among the most common genetic diseases worldwide. Current approaches to the development of murine models of SCA involve the elimination of functional murine α- and β-globin genes and substitution with human α and βs transgenes. Recently, two groups have produced mice that exclusively express human HbS. The transgenic lines used in these studies were produced by coinjection of human α-, γ-, and β-globin constructs. Thus, all of the transgenes are integrated at a single chromosomal site. Studies in transgenic mice have demonstrated that the normal gene order and spatial organization of the members of the human β-globin gene family are required for appropriate developmental and stage-restricted expression of the genes. As the cis-acting sequences that participate in activation and silencing of the γ- and β-globin genes are not fully defined, murine models that preserve the normal structure of the locus are likely to have significant advantages for validating future therapies for SCA. To produce a model of SCA that recapitulates not only the phenotype, but also the genotype of patients with SCA, we have generated mice that exclusively express HbS after transfer of a 240-kb βs yeast artificial chromosome. These mice have hemolytic anemia, 10% irreversibly sickled cells in their peripheral blood, reticulocytosis, and other phenotypic features of SCA.
The biochemical basis for sickle cell anemia (SCA) was described more than 30 years ago (1, 2); however, advances in the treatment of SCA have in part been hampered by the lack of an animal model that accurately reproduces the pathophysiology and genetics of this disorder. The strategies for making a murine model of SCA have evolved as the limitations of each approach became apparent. Thus, initial efforts focused on the transfer of human βs-globin genes to generate transgenic lines. Heterotetramers of murine α-globin and human βs-globin do not polymerize efficiently; even with the addition of human α-globin transgenes only a small fraction of the cells sickled in vivo because of the disruption of HbS by murine α- and β-globins (3–11). Under hypoxic conditions there is more extensive deoxygenation of murine hemoglobin than HbS, as mouse hemoglobin has a lower O2 affinity than does HbS (12). To produce a hemoglobin that would polymerize more readily, two additional mutations were introduced into βs transgenes; a second mutation in codon 23 to reproduce the βs Antilles allele, and a third mutation to yield βs AntillesD Punjab or HbSAD (6, 7, 13). While the SAD mice exhibited a greater propensity for red cell sickling under hypoxic conditions, this model did not fully recapitulate the features of sickle cell disease. Hence, recent efforts have been directed toward reducing or eliminating endogenous mouse globin gene expression and the production of mice that exclusively express human HbS as adults.
Two such models have been reported recently (15, 16). Expression of the adult murine α- and β-globin genes was eliminated by targeted disruption of these loci in embryonic stem (ES) cells. Mice exclusively expressing human HbS were identified after successive cycles of crossbreeding knockout lines with lines expressing human α- and βs-globin transgenes. Both of these SCA models exhibit anemia, sickling of peripheral red blood cells, and the presence of irreversibly sickled cells, as well as organ pathology (15, 16). The transgenic lines expressing human α-, γ-, and β-globin were produced by coinjection of fragments containing sequences from the β-globin locus control region (LCR), and human α-, γ-, and β-globin genes. Thus, the human α-, γ-, and β-globin transgenes are integrated at a single chromosomal site.
Previous studies of transgenic mice generated with plasmid, cosmid, and yeast artificial chromosome (YAC) constructs containing human β-globin genes have demonstrated that the normal sequence context and native order of the genes are required for appropriately regulated developmental stage and tissue-specific expression (17–21). We and others have shown that the developmental pattern of expression of the human ɛ-, γ-, and β-globin genes in transgenic mice generated by YAC transfer is highly reproducible, regardless of the position of integration of the wild-type locus (22). Murine models that preserve the integrity of the entire human β-globin locus may have significant advantages in determining the efficacy of future strategies such as homologous recombination and reactivation of γ-globin gene expression. To produce a murine model of SCA that recapitulates not only the phenotype but also the genetic locus encompassing βs-globin gene in patients with SCA, we have transferred a 240-kb βs-globin YAC in which members of the human β-globin gene cluster are present in their native genomic context. To produce mice that express only HbS, the βs-globin YAC transgenic mice were bred onto a murine βo thalassemic background (Hbbth-3) (23) and subsequently crossbred with mice heterozygous for murine α1- and α2-globin gene deletions and expressing human α-globin. These animals are viable, show irreversibly sickled cells in their peripheral blood smears, and have hemolytic anemia. Sickle cell mice harboring a βs-globin YAC provide a novel model for assessing future therapies for SCA.
MATERIALS AND METHODS
Mice with Targeted Disruptions of the Murine Adult α- and β-Globin Genes.
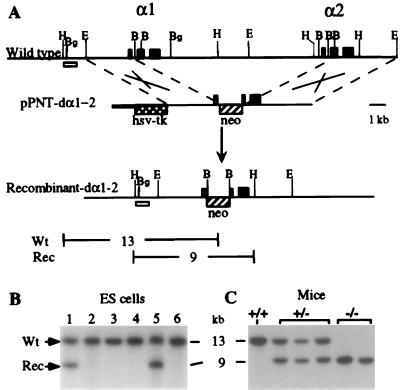
Mice carrying a deletion of the βmaj and βmin-globin genes (Hbbth-3) produced by homologous recombination were generously provided by Oliver Smithies, University of North Carolina at Chapel Hill (23). To generate mice with targeted deletion of the murine adult α1- and α2-globin genes, a 4.2-kb EcoRI-BamHI fragment, including exon 1 and 5′ flanking sequences of the murine α1-globin gene, was inserted between the herpes simplex thymidine kinase (HSV-TK) gene and the neophosphotransferase (neo) cassette of the pPNT vector provided by Richard Mulligan (24). A 4.8-kb BamHI-EcoRI fragment including the 3′ end of exon 2 and extending to 3′ flanking sequences of the α2-globin gene was inserted 3′ to the neo gene. This vector was electroporated into ES cells, followed by selection in G418 and ganciclovir. Homologous recombinants were identified by Southern analysis after digestion of genomic DNA from individual ES clones with HindIII (Fig. 1). The targeted ES cells were introduced into C57BL/6 blastocysts to produce chimeras. Germ-line transmission was demonstrated by breeding these chimeras onto the C57BL/6 background. F1 mice heterozygous for the deletion of the murine adult α1- and α2-globin genes then were used in subsequent breeding steps.
Figure 1.
One-step targeted disruption of the murine α1- and α2-globin gene loci. (A) The genomic arrangement of the mouse α-globin loci is shown on the first line; pPNT-dα1–2 is the targeting vector containing the hsv-tk and neo genes. The arrow points to the structure of the recombinant disrupted α1–2 structure. The open rectangle indicates the probe used to detect the 13-kb HindIII fragment in the wild type (WT) and the 9 kb in the disrupted (Rec) allele. Southern analysis of WT and targeted ES cells (B) and of normal and mice heterozygous and homozygous for α-globin gene Knockout (C) are shown.
Transgenic Lines Carrying the Human α2-Globin Gene or the βs YAC.
A transgenic line carrying two copies of the human α2-globin gene linked to a 6.5-kb mini-LCR (25) was generated on a C57BL/6/SJL hybrid background and was provided by Stephen Liebhaber (University of Pennsylvania). Mice carrying the βs-globin YAC were produced as follows. To facilitate future genetic manipulation, the 230-kb β-globin YAC in strain A85D10 (28) was modified by disruption of the URA 3 gene in the right (3′) arm by the insertion of the LYS 2-neo gene cassette to yield A85.D10 neo/lys (26, 27). A URA 3 containing yeast integrating plasmid (yIP) RK306-βs was generated by inserting a 2-kb EcoRI fragment (GenBank, HUMHBB 61537–63548) from pLAR-βs containing human βs-globin gene sequences (provided by Mark Groudine, Fred Hutchinson Cancer Research Institute), into the RK306 yIP (provided by Joakim Li, University of California, San Francisco). The RK306- βs vector was linearized by digestion with BamHI and electroporated into the A85D10 neo/lys yeast strain. After selection in Ura, Trp, and Lys media, colonies were purified and screened by PCR amplification of a 534-bp fragment of the β-globin gene by using the following oligonucleotide primers 5′ (5′-GTACGGCTGTCATCACTTAGACCTCA-3′) and 3′ (5′-GCCATCACTAAAGGCACCG-3′). The amplified product encompassing the codon 6 A-to-T transversion was digested with DdeI (Fig. 2A). Negative selection in medium containing 5-fluoroorotic acid selects for the eviction of the URA containing plasmid. PCR and Southern analysis were used to identify strains carrying the intact 240-kb βs-globin YAC as described (19). The βs YAC subsequently was purified by pulsed-field gel electrophoresis and excised from agarose gels as described (19).
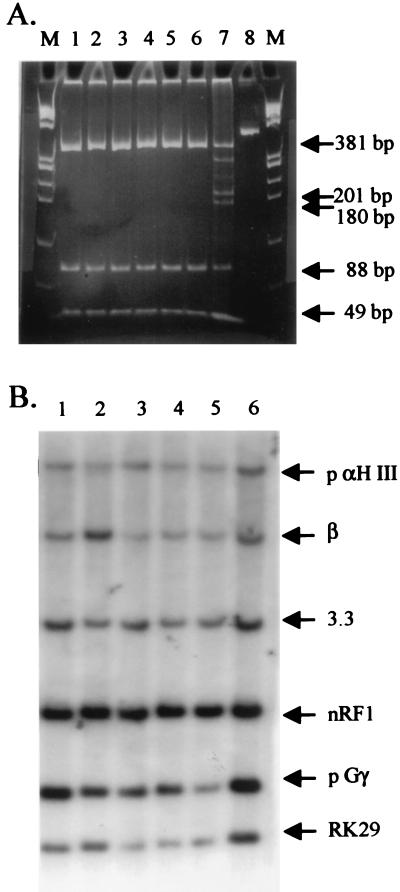
Figure 2.
Analysis of βs YAC DNA and βs YAC transgenic mice. A shows the products of DdeI digestion of a 534-bp fragment after PCR amplification of DNA from six yeast clones identified by genetic screening. The fragment was digested with DdeI and fractionated on a 5% acrylamide gel. Normal restriction fragments are 49, 88, 180, and 201 bp in length. The codon 6 A-to-T transversion results in the loss of a DdeI site and the appearance of a 381 (180+201)-bp fragment. Lanes 1–6: DdeI-restricted DNA from six independent yeast clones containing the β-globin YAC with the βs mutation. Lane 7: DdeI-restricted PCR-amplified DNA from an individual with sickle trait (A/S). The normal restriction fragments as well as the 381-bp mutant fragment are observed. Two additional bands present in this lane are the products of partial digestion. Lane 8: Undigested PCR-amplified yeast DNA demonstrating the 534-bp full-length fragment. Lanes M: φx174 HaeIII marker DNA ladder. (B) Southern analysis of EcoRI-restricted genomic DNA from transgenic line βs.32 (lanes 1 and 6) and from four independent transgenic lines carrying intact copies of the wild-type β-globin YAC sequences (lanes 2–5). The correct fragments hybridizing with the γ- (pGγ) and β-globin genes, LCR-hypersensitive site 3 (PaH III), upstream LCR sequences (3.3R1), and sequences 20 kb downstream of the β-globin gene (RK29) are present in all of the lanes.
Production of βs YAC Transgenic Mice.
FVBn1 embryos were injected with the purified βs YAC and transferred according to standard protocols (29). Founder mice were screened by using multiplex PCR. The structure of the integrated YAC sequences was analyzed by conventional and long-range restriction mapping as described (19). In addition, the βs genomic DNA in transgenic line βs.32 and βs cDNA from this line were sequenced.
Primer Extension Analysis.
The expression of the human β-globin gene family in line βs.32 was analyzed in yolk sac on days 8.5–11.5 of gestation, in fetal liver on days 12.5–18.5, and in adult peripheral blood samples and quantitated by PhosphorImager analysis as described (19).
Hematological Parameters.
Complete blood counts were performed on a Celldyne 3500 apparatus (Idexx Veterinary Services, West Sacramento, CA). Peripheral red blood cell sickling was assessed in wet mounts of peripheral blood after incubation for 15 min with an equal volume of 2% sodium metabisulfite (30).
Hemoglobin Analysis.
Cellulose acetate gel electrophoresis was performed on peripheral blood samples from normal mice and sickle βs YAC mice [Helena Laboratories (31)]. Globin chains were analyzed by Triton/urea gel electrophoresis (32).
RESULTS
Our strategy for producing mice that express only HbS as adults and preserve the integrity of the human β-globin locus was to crossbreed four different lines of mice. These lines were (i) mice carrying a targeted deletion of the α1- and α2-globin genes; (ii) mice with a targeted deletion of the adult murine β-globin genes; (iii) mice carrying the human α-globin transgene, and (iv) mice with the human βs YAC transgenic locus. As human α-globin transgenic mice and mice with βmin- and βmaj-globin genes were available to us, we undertook the generation of targeted deletion of the murine α-globin genes and production of βs YAC transgenic mice.
Mouse α-Globin Gene Disruption and Rescue of α Thalassemic Mice by α-Globin Transgenes.
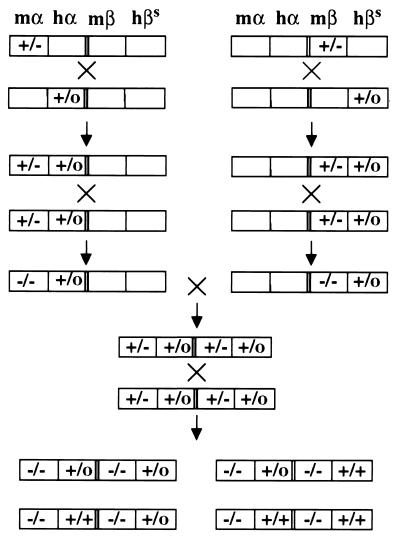
Disruption of the mouse α-globin genes was accomplished by using the pPNT-dα1–2 vector described in Fig. 1A. Six out of 72 neo-resistant ES cell clones had undergone the correct targeting event and were identified by the presence of the 9-kb targeted allele (Fig. 1B, lanes 1 and 5). Two independent cell lines were used to generate chimeric mice. Subsequent breeding generated mice with targeted mutations (Fig. 1C). Mice homozygous and heterozygous for the deletion of both murine α-globin genes were designated mα−/− and mα+/−, respectively. Mα+/− mice were bred with transgenic mice carrying the human LCR-α2-globin genes (hα+/o). Subsequently, mice that were heterozygous for the mouse α-globin knockout and hemizygous for the hα2 transgene (mα+/−, hα+/o) were interbred to produce mice that were homozygous for the deletion of murine α1 and α2 and expressed only human α2 (mα−/−, hα+/o) (Fig. 3). Viable mice were produced with this genotype, demonstrating that the level of human α-globin is sufficient to rescue mice from lethal α thalassemia as seen by others (33).
Figure 3.
Breeding scheme to generate the SCA mouse. The four different loci in the mice are depicted by the four boxes. Heterozygous and homozygous knockouts of the mouse α (mα) and mouse β (mβ) are indicated by +/− and −/−, and hemizygous and homozygous transgenes of the human α (ha) and human βs (hβs) are indicated by +/o and +/+, respectively.
Production of βs YAC Transgenic Mice and Rescue of β Thalassemic Line Hbbth-3.
To construct a β-globin YAC carrying the βs mutation we used a 230-kb β-globin YAC (A85.D10) that was modified initially by one-step homologous recombination to disrupt the URA gene and insert the LYS 2 gene and the neogene (A85.D10 neo/lys). The A85D10 neo/lys yeast strain then was subjected to two-step recombination with vector RK306-βs. Homologous recombinants were identified by genetic screening and PCR amplification of a 534-bp fragment spanning the βs mutation. The A-to-T transversion of the βs gene results in the loss of a DdeI site (34) and the appearance of a 381-bp fragment on acrylamide gels (Fig. 2A, lanes 1–6). After purification and microinjection of the βs-globin YAC, transgenic line βs.32 was identified. Conventional and long-range restriction mapping demonstrated fragments of the correct size hybridized with probes for each of the genes of the locus, as well as for sequences in the LCR and 5′ and 3′ flanking regions of the locus (Fig. 2B). PhosphorImager analysis of Southern blots after these hybridizations indicated that there are two copies of the βs YAC. The level of βs mRNA expression in adult animals was 40% of mouse β-globin per gene copy by primer extension analysis, and the developmental pattern of gene expression in line βs.32 was very similar to that observed in wild-type YAC transgenic lines (data not shown and ref. 22).
The βs.32 line (designated hβs) was bred with the Hbbth-3 line with a targeted deletion of both the murine βmaj and βmin-globin genes (mβ+/−) (23). Mice homozygous for this deletion (mβ−/−) are rescued by the presence of the βs-globin YAC transgenic locus in line βs.32.
Production of a Model of Sickle Cell Anemia.
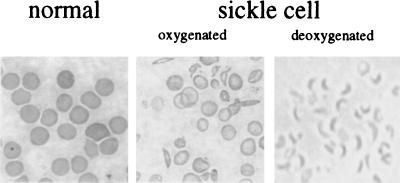
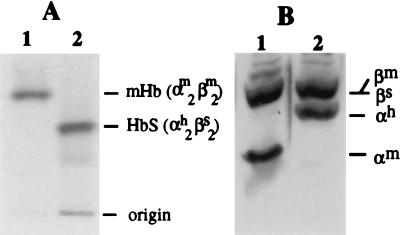
The (mβ−/−, hβs+/o) mice were bred with (mα−/−, hα+/o) animals to produce mice heterozygous for mouse α- and β-globin gene knockouts and hemizygous for human α- and β-globin transgenes (mα−/−, hα +/o, mβ−/−, hβs+/o). Successive cycles of breeding were carried out with these mice, to produce animals expressing only HbS (Fig. 3). Such mice could have one of four genotypes shown in Fig. 3, and the expected frequency of obtaining a mouse expressing only HbS is 1/64 pups. However, we were only able to obtain such a mouse at a frequency of 1/500 pups examined. This indicates that mice expressing only HbS may have difficulty surviving in utero or the first days of life or are cannibalized early in the neonatal period. When compared with normal C57BL/6 mice, animals exclusively expressing human HbS are anemic (Hct 22.4%, Table 1). They have approximately 10% irreversibly sickled cells in their peripheral blood, and >80% of red blood cells show HbS polymerization and sickling in the presence of sodium metabisulfite (Table 1 and Fig. 4 A and B). Hemoglobin analysis by cellulose acetate electrophoresis confirmed the presence of only human HbS, and triton gel electrophoresis showed only human α- and βs-globin in the peripheral blood (Fig. 5A and B).
Table 1.
Hematological data in peripheral blood of normal and SCA mouse
| Mouse | Red blood cells, 106/ml | Hemoglobin, g/dl | Hematocrit, % | Mean corpuscular volume, fl | Mean corpuscular hemoglobin, pg | Mean corpuscular hemoglobin concentration, g/dl | Nucleated red blood cells per 100 white blood cells | Reticulocyte, % |
|---|---|---|---|---|---|---|---|---|
| Normal* | 9.8 ± 0.4 | 15.7 ± 0.4 | 47.3 ± 1.7 | 48.8 ± 0.8 | 16.1 ± 0.4 | 33.1 ± 0.9 | 0 | 1.1 ± 0.2 |
| SCA | 5.1 | 5.3 | 22.4 | 44 | 10.4 | 23.9 | 10 | 20 |
Normal values were derived from average of four samples.
Figure 4.
Photomicrographs of mouse blood smears. (Left and Center) Wright stain of blood from normal and sickle cell anemia mouse; note the irreversible sickle form seen in the oxygenated blood of the latter. Right shows the sickle mouse red cell deoxygenated with sodium metabisulfite.
Figure 5.
Electrophoresis of hemolysates from the normal and sickle cell anemia mouse. (A) Cellulose acetate electrophoresis of hemoglobin. (B) Triton-urea gel electrophoresis of globin chain.
DISCUSSION
We have demonstrated that mice with LCR hα2 and βs YAC transgenes rescue knockout lines with homozygous deletions of the adult murine α- and β-globin genes. Mice exclusively expressing HbS in this model have the phenotype of irreversibly sickled cells in the peripheral blood, anemia, and reticulocytosis observed in other murine models of sickle cell disease and in individuals with SCA. The developmental expression of the human β-globin gene family is comparable to the pattern observed in transgenic lines carrying the wild-type locus. Thus, γ-globin gene expression decreases to 1–5% at birth and is undetectable in mature mice carrying the βs YAC.
There are several possible explanations for the low frequency of live-born mice exclusively expressing HbS. First, the pattern of expression of the human γ-globin genes in YAC transgenic mice differs substantially from their expression in human development. βs YAC transgenic mice express relatively high levels of human γ-globin until days 12–14 of gestation. Thereafter, the level of γ-globin at the mRNA and protein levels decreases to 1–5% at birth. Thus, mice expressing HbS are not protected from intrauterine and perinatal sickling crises during hypoxic periods as are human infants with SCA who express high levels of HbF during the perinatal period. It is likely that there is a high rate of perinatal wastage of fetuses expressing HbS in our model. In contrast, in both of the recently described transgenic/knockout models of SCA, γ-globin levels in newborn mice ranged from 30 to 50% in one model (15) and 4 to 26% in the other (16). In the latter study, a cohort of newborn mice dying in the first few hours of life was described. The death of these pups was attributed to hypoxia and the sequelae of intravascular red cell sickling in the lungs and other tissues (16).
A second factor limiting the survival of pups exclusively expressing HbS may be attributed to variation in independently segregating factors. These may be proteins that participate in the regulation of gene expression or play a role in as yet undefined regulatory pathways. It is clear from analysis of human populations with SCA and murine models of SCA that epigenetic factors contribute to the severity of sickle cell disease. For example, independent loci that participate in the phenotype of hereditary persistence of fetal hemoglobin have been mapped to chromosome 6 (35) and to the X chromosome (36). The identification of other genetic loci that modulate the severity of SCA will have important implications for the development of future therapeutic approaches for this disorder.
There are several potential advantages of our sickle cell mice harboring the βs YAC for future studies evaluating new therapeutic approaches for the treatment of SCA. Although some factors that moderate the severity of sickle cell disease are known, others are not yet defined and their site of interaction with the β-globin locus is not known. Thus, it is important to preserve the sequence context and the spatial organization of the β-like genes and LCR. Similarly, the cis-acting sequences and trans-acting factors that participate in activation and silencing of the human γ-globin genes also are not completely understood. Future therapeutic options for SCA almost certainly will include efforts to develop drugs that reactivate fetal globin gene expression. The normal genomic context of the γ-globin genes may be an important prerequisite for responses to drugs in murine models to be predictive of their effects in human erythroid cells and progenitors. Finally, homologous recombination using oligonucleotides or other targeting constructs may develop as a viable therapeutic strategy (37). Clearly, the efficiency of homologous recombination in vivo using different targeting constructs will be evaluated best when the chromosomal locus containing the βs-globin gene retains its native structure and conformation as it does in YAC transgenic animals.
Acknowledgments
We thank Oliver Smithies and Stephen Liebhaber for knockout and transgenic lines, respectively, and Mark Groudine, Richard Mulligan, and Joachim Li for plasmids. This work was supported by National Institutes of Health Grant HL53762 and the transgenic facility at the University of California, San Francisco, by the Lucille Markey Foundation. Y.W.K is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- SCA
sickle cell anemia
- YAC
yeast artificial chromosome
- ES
embryonic stem
- LCR
locus control region
- neo
neophosphotransferase
References
- 1.Pauling L, Itano H A, Singer S J, Wells I C. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 2.Ingram V M. Nature (London) 1956;178:792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- 3.Ryan T M, Townes T M, Reilly M P, Asakura T, Palmiter R D, Brinster R L, Behringer R R. Science. 1990;247:566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- 4.Greaves D R, Fraser P, Vidal M A, Hedges M J, Ropers D, Luzzatto L, Grosveld F. Nature (London) 1990;343:183–185. doi: 10.1038/343183a0. [DOI] [PubMed] [Google Scholar]
- 5.Fabry M E, Sengupta A, Suzuka S M, Costantini F, Rubin E M, Hofrichter J, Christoph G, Manci E, Culberson D, Factor S M, et al. Blood. 1995;86:2419–2428. [PubMed] [Google Scholar]
- 6.Trudel M, Saadane N, Garel M C, Bardakdjian-Michau J, Blouquit Y, Guerquin-Kern J L, Rouyer-Fessard P, Vidaud D, Pachnis A, Roméo P H, et al. EMBO J. 1991;10:3157–3165. doi: 10.1002/j.1460-2075.1991.tb04877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trudel M, De Paepe M E, Chrétien N, Saadane N, Jacmain J, Sorette M, Hoang T, Beuzard Y. Blood. 1994;84:3189–3197. [PubMed] [Google Scholar]
- 8.Fabry M E, Kennan R P, Paszty C, Costantini F, Rubin E M, Gore J C, Nagel R L. J Clin Invest. 1996;98:2450–2455. doi: 10.1172/JCI119062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabry M E, Costantini F, Pachnis A, Suzuka S M, Bank N, Aynedjian H S, Factor S M, Nagel R L. Proc Natl Acad Sci USA. 1992;89:12155–12159. doi: 10.1073/pnas.89.24.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popp R A, Popp D M, Shinpock S G, Yang M Y, Mural J G, Aguinaga M P, Kopsombut P, Roa P D, Turner E A, Rubin E M. Blood. 1997;89:4204–4212. [PubMed] [Google Scholar]
- 11.Rhoda M D, Domenget C, Vidaud M, Bardakdjian-Michau J, Rouyer-Fessard P, Rosa J, Beuzard Y. Biochim Biophys Acta. 1988;952:208–12. doi: 10.1016/0167-4838(88)90117-3. [DOI] [PubMed] [Google Scholar]
- 12.D’Surney S J, Popp R A. Genetics. 1992;132:545–551. doi: 10.1093/genetics/132.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monplaisir N, Merault G, Poyart C, Rhoda M D, Craescu C, Vidaud M, Galacteros F, Blouquit Y, Rosa J. Proc Natl Acad Sci USA. 1986;83:9363–9367. doi: 10.1073/pnas.83.24.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin E M, Witkowska H E, Spangler E, Curtin P, Lubin B H, Mohandas N, Clift S M. J Clin Invest. 1991;87:639–647. doi: 10.1172/JCI115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan T M, Ciavatta D J, Townes T M. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 16.Pászty C, Brion C M, Manci E, Witkowska H E, Stevens M E, Mohandas N, Rubin E M. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 17.Strouboulis J, Dillon N, Grosveld F. Genes Dev. 1992;6:1857–1864. doi: 10.1101/gad.6.10.1857. [DOI] [PubMed] [Google Scholar]
- 18.Peterson K R, Stamatoyannopoulos G. Mol Cell Biol. 1993;13:4836–4843. doi: 10.1128/mcb.13.8.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaensler K M, Kitamura M, Kan Y W. Proc Natl Acad Sci USA. 1993;90:11381–11385. doi: 10.1073/pnas.90.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillon N, Strouboulis J, Grosveld F. In: The Regulation of Human β-Globin Gene Switching. Stamatoyannopoulos G, editor. London: U.K. Intercept; 1995. pp. 21–28. [Google Scholar]
- 21.Kollias G, Wrighton N, Hurst J, Grosveld F. Cell. 1986;46:89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- 22.Porcu S, Kitamura M, Witkowska E, Zhang Z, Mutero A, Lin C, Chang J, Gaensler K M. Blood. 1997;90:4602–4609. [PubMed] [Google Scholar]
- 23.Detloff P J, Lewis J, John S W, Shehee W R, Langenbach R, Maeda N, Smithies O. Mol Cell Biol. 1994;14:6936–6943. doi: 10.1128/mcb.14.10.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 25.Talbot D, Collis P, Antoniou M, Vidal M, Grosveld F, Greaves D R. Nature (London) 1989;338:352–355. doi: 10.1038/338352a0. [DOI] [PubMed] [Google Scholar]
- 26.Rothstein R J. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 27.Brownstein B H, Silverman G A, Little R D, Burke D T, Korsmeyer S J, Schlessinger D, Olson M V. Science. 1989;244:1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- 28.Gaensler K M, Burmeister M, Brownstein B H, Taillon-Miller P, Myers R M. Genomics. 1991;10:976–984. doi: 10.1016/0888-7543(91)90188-k. [DOI] [PubMed] [Google Scholar]
- 29.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 219–252. [Google Scholar]
- 30.Daland G A, Castle W B. J Lab Clin Med. 1948;33:1082–1088. [PubMed] [Google Scholar]
- 31.Whitney J B D. Biochem Genet. 1978;16:667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]
- 32.Rovera G, Magarian C, Borun T W. Anal Biochem. 1978;85:506–518. doi: 10.1016/0003-2697(78)90248-8. [DOI] [PubMed] [Google Scholar]
- 33.Pászty C, Mohandas N, Stevens M E, Loring J F, Liebhaber S A, Brion C M, Rubin E M. Nat Genet. 1995;11:33–39. doi: 10.1038/ng0995-33. [DOI] [PubMed] [Google Scholar]
- 34.Chang J C, Kan Y W. Lancet. 1981;2:1127–1129. doi: 10.1016/s0140-6736(81)90584-5. [DOI] [PubMed] [Google Scholar]
- 35.Garner C, Mitchell J, Hatzis T, Reittie J, Farrall M, Thein S L. Am J Human Genet. 1998;62:1468–1474. doi: 10.1086/301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y P, Maier-Redelsperger M, Smith K D, Contu L, Ducroco R, de Montalembert M, Belloy M, Elion J, Dover G J, Girot R. Brit J Haematol. 1997;96:806–814. doi: 10.1046/j.1365-2141.1997.d01-2094.x. [DOI] [PubMed] [Google Scholar]
- 37.Caplen N J, Kinrade E, Sorgi F, Gao X, Gruenert D, Geddes D, Coutelle C, Huang L, Alton E W, Williamson R. Gene Ther. 1995;2:603–613. [PubMed] [Google Scholar]