Abstract
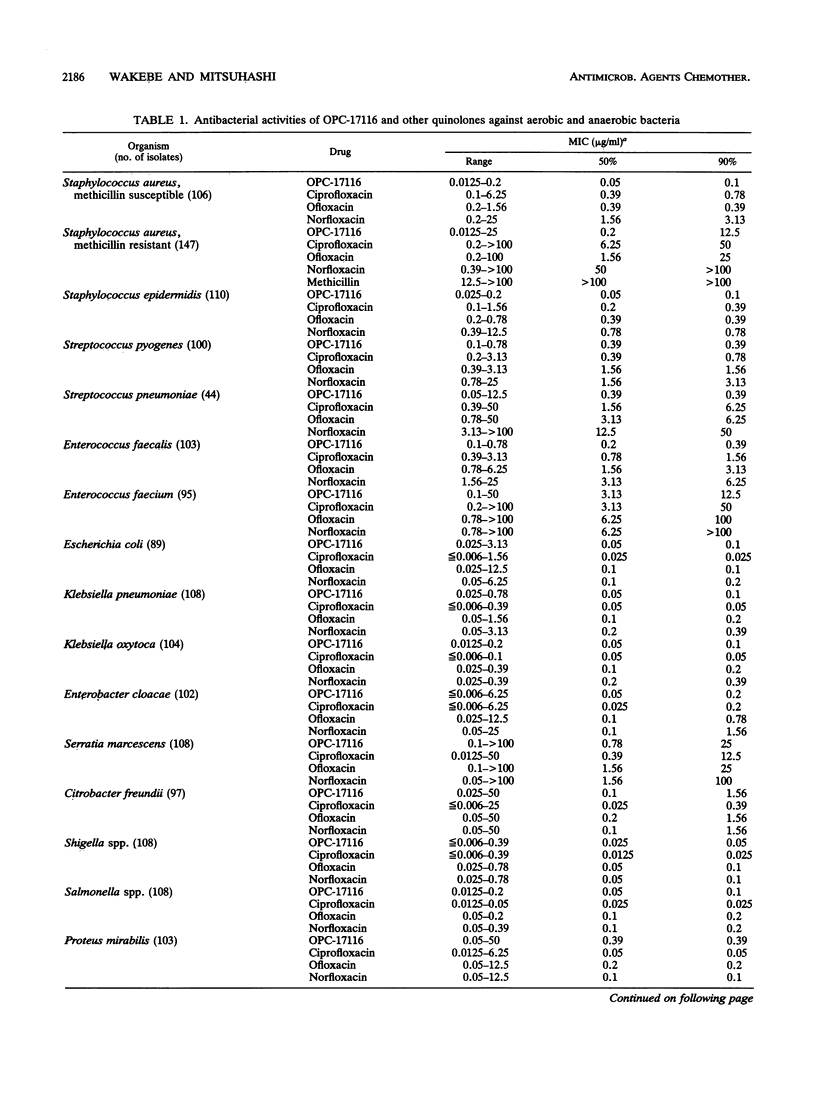
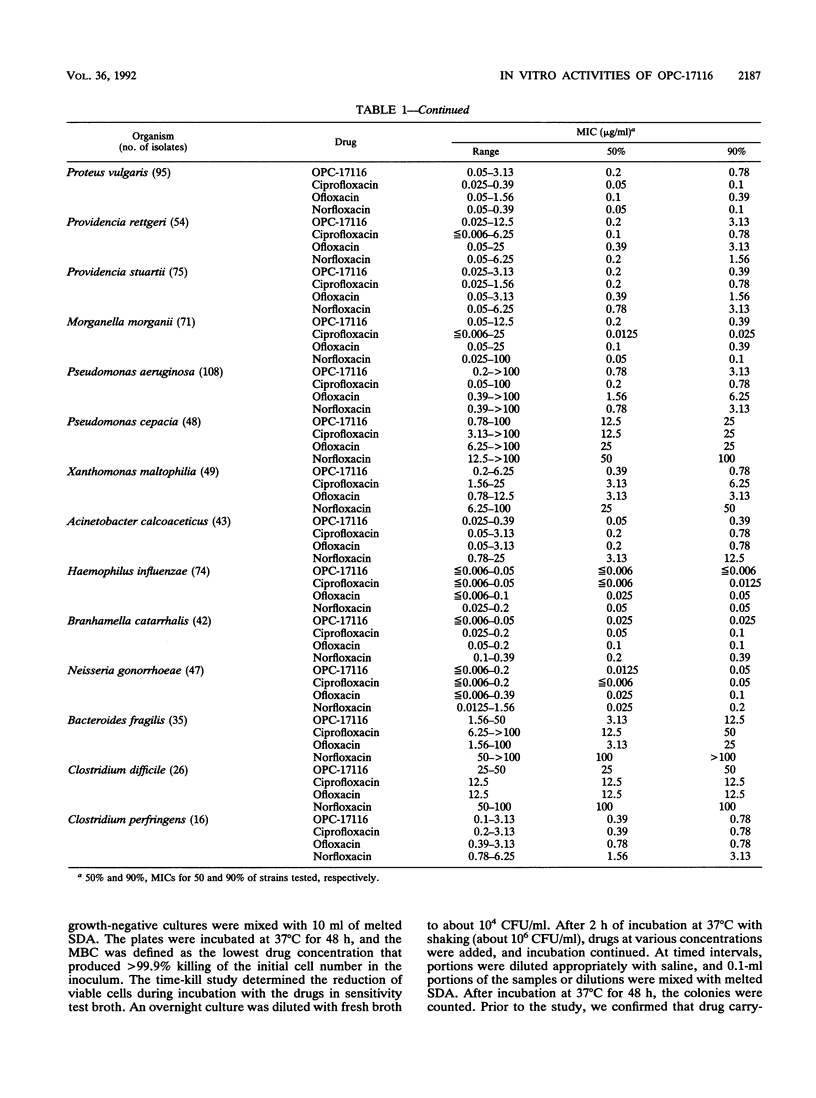
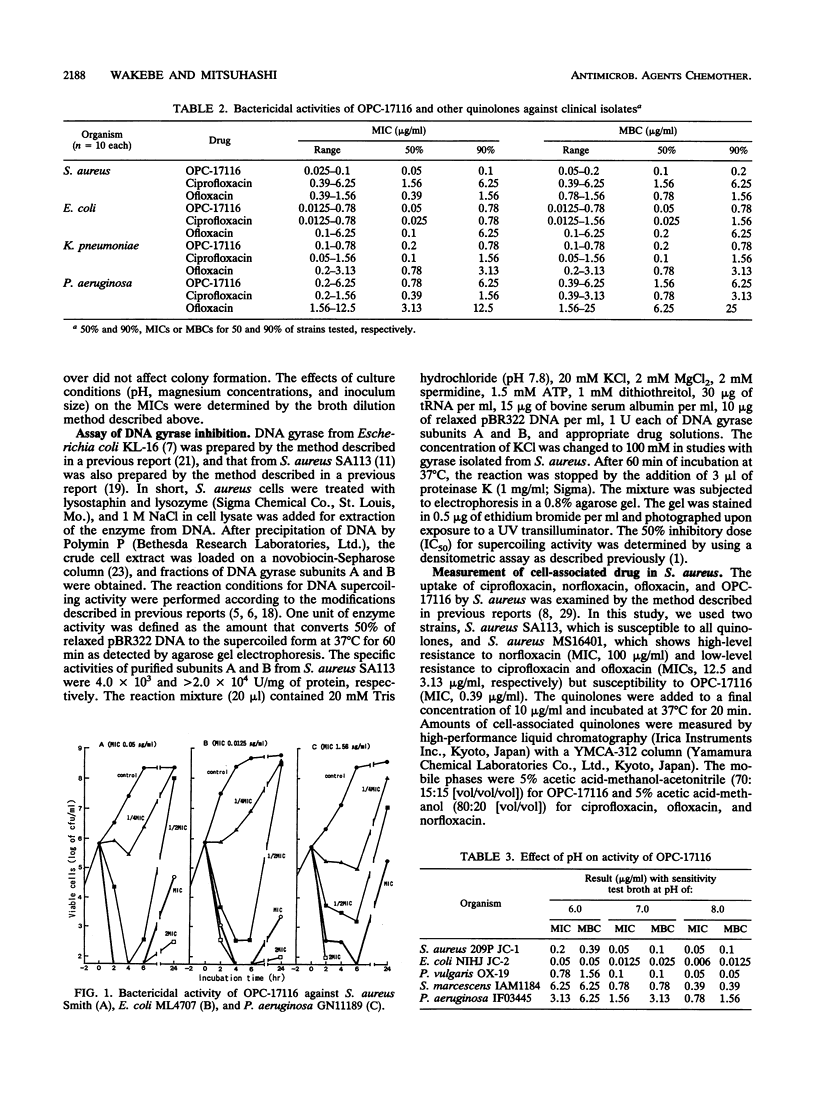
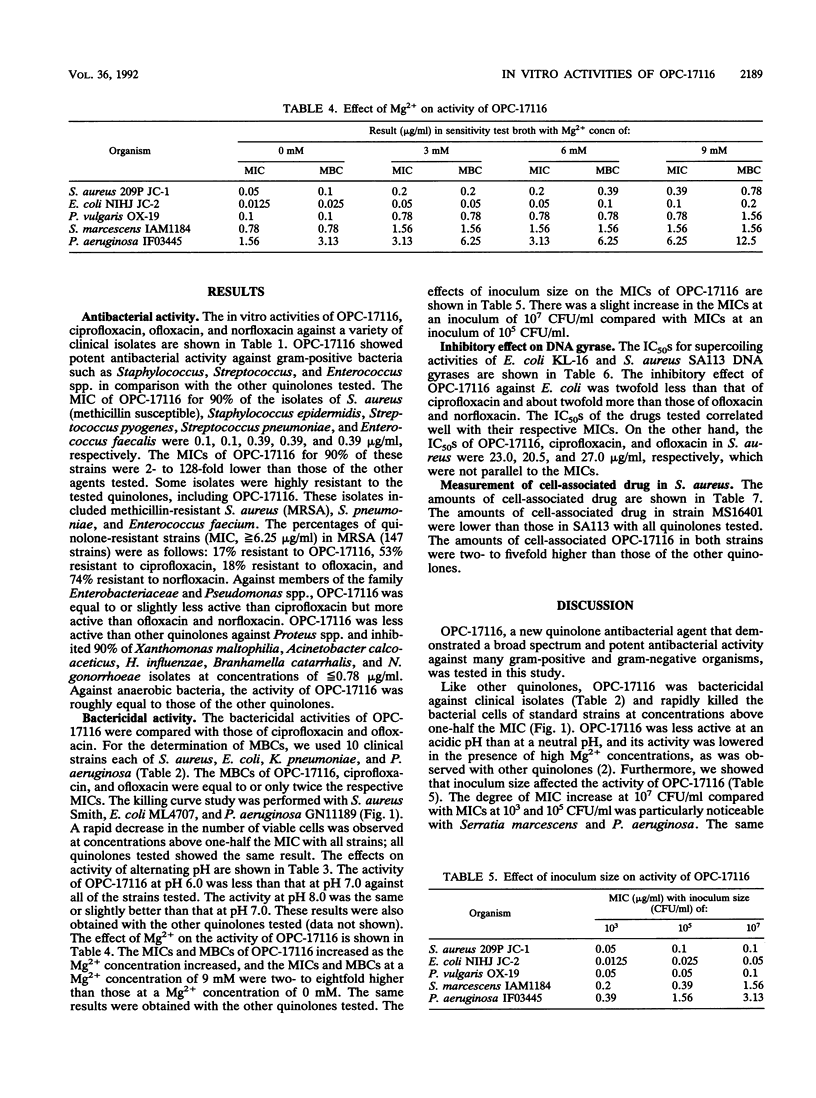
The in vitro antibacterial activity of OPC-17116, a new fluoroquinolone, against a wide variety of clinical isolates was evaluated and compared with those of ciprofloxacin, ofloxacin, and norfloxacin. OPC-17116 showed potent broad-spectrum activity against gram-positive and -negative bacteria. The activity of this compound against gram-positive bacteria was higher than those of other quinolones, and its activity against gram-negative and anaerobic bacteria was roughly comparable to those of other quinolones. OPC-17116 had potent activity against important pathogens of respiratory tract infections such as Staphylococcus aureus, Streptococcus pneumoniae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, and Branhamella catarrhalis. The MICs of this compound against 90% of these organisms, except for methicillin-resistant S. aureus, ranged from less than or equal to 0.006 to 3.13 micrograms/ml. OPC-17116 at more than one-half the MICs was bactericidal against clinical isolates of S. aureus, Escherichia coli, K. pneumoniae, and P. aeruginosa. The activity of OPC-17116 was decreased by several culture conditions such as acidic pH, high concentration of Mg2+ ions, and inoculum size of 10(7) CFU/ml. OPC-17116 inhibited the supercoiling activity of DNA gyrases from E. coli KL-16 and S. aureus SA113 (50% inhibitory concentrations, 0.19 and 23.0 micrograms/ml, respectively). The amount of OPC-17116 accumulation was higher than that of other quinolones in S. aureus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Sato K., Fujii T., Fujimaki K., Inoue M., Mitsuhashi S. Purification of Citrobacter freundii DNA gyrase and inhibition by quinolones. Antimicrob Agents Chemother. 1988 Jan;32(1):104–109. doi: 10.1128/aac.32.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Novelli A., Neu H. C. In vitro activity of lomefloxacin (SC-47111; NY-198), a difluoroquinolone 3-carboxylic acid, compared with those of other quinolones. Antimicrob Agents Chemother. 1988 May;32(5):656–662. doi: 10.1128/aac.32.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum T. E., Schaberg D. R., Terpenning M. S., Sottile W. S., Kauffman C. A. Increasing resistance of Staphylococcus aureus to ciprofloxacin. Antimicrob Agents Chemother. 1990 Sep;34(9):1862–1863. doi: 10.1128/aac.34.9.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Fisher L. M., O'Dea M. H. DNA gyrase: purification and catalytic properties of a fragment of gyrase B protein. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6289–6293. doi: 10.1073/pnas.76.12.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Mode of action of the quinolone antimicrobial agents. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S14–S21. doi: 10.1093/clinids/10.supplement_1.s14. [DOI] [PubMed] [Google Scholar]
- Imada T., Miyazaki S., Nishida M., Yamaguchi K., Goto S. In vitro and in vivo antibacterial activities of a new quinolone, OPC-17116. Antimicrob Agents Chemother. 1992 Mar;36(3):573–579. doi: 10.1128/aac.36.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu S., Surdeanu M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J Gen Microbiol. 1976 Oct;96(2):277–281. doi: 10.1099/00221287-96-2-277. [DOI] [PubMed] [Google Scholar]
- Ito A., Hirai K., Inoue M., Koga H., Suzue S., Irikura T., Mitsuhashi S. In vitro antibacterial activity of AM-715, a new nalidixic acid analog. Antimicrob Agents Chemother. 1980 Feb;17(2):103–108. doi: 10.1128/aac.17.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T., Inoue M., Mitsuhashi S. In vitro activity of AT-4140 against clinical bacterial isolates. Antimicrob Agents Chemother. 1989 Nov;33(11):1980–1988. doi: 10.1128/aac.33.11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. Differing activities of quinolones against ciprofloxacin-susceptible and ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):345–350. doi: 10.1128/aac.35.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Yoshida S., Wakebe H., Inoue M., Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Jun;35(6):1053–1059. doi: 10.1128/aac.35.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Yoshida S., Wakebe H., Inoue M., Yamaguchi T., Mitsuhashi S. Mechanisms of clinical resistance to fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Dec;35(12):2562–2567. doi: 10.1128/aac.35.12.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Matsuura Y., Inoue M., Une T., Osada Y., Ogawa H., Mitsuhashi S. In vitro and in vivo activity of DL-8280, a new oxazine derivative. Antimicrob Agents Chemother. 1982 Oct;22(4):548–553. doi: 10.1128/aac.22.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Sato K., Kimura Y., Hayakawa I., Osada Y., Nishino T. Inhibition by quinolones of DNA gyrase from Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Jul;35(7):1489–1491. doi: 10.1128/aac.35.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend D. E., Ashdown N., Bolton S., Bradley J., Duckworth G., Moorhouse E. C., Grubb W. B. The international spread of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1987 Jan;9(1):60–71. doi: 10.1016/0195-6701(87)90097-1. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Itoh-Yamashita N., Konno M. Cloning and expression of the norA gene for fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1989 Sep;33(9):1535–1539. doi: 10.1128/aac.33.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990 Dec;172(12):6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kojima T., Inoue M., Mitsuhashi S. Uptake of sparfloxacin and norfloxacin by clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):368–370. doi: 10.1128/aac.35.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


