Abstract
Antileishmanial chemotherapy is hampered by the location of the parasite within the phagolysosome of the macrophage, which restricts the bioavailability of many potentially useful antileishmanial drugs. In this study, the possibility of using antileishmanial drugs targeted to the infected macrophages by means of a chemical linkage to a neutral mannose-substituted poly-L-lysine carrier molecule was explored. The study was performed in an in vitro model with Leishmania donovani-infected murine macrophages. The antileishmanial activities of various synthetic constructs were compared with those of the free drugs and the pentavalent antimonial Pentostam, which was used as the positive control. The 50% effective dose of allopurinol riboside linked to the mannosylated poly-L-lysine was below 7.5 x 10(-6) M, while it was up to 3 x 10(-4) M for the free drug, indicating that the drug bound to the polymer was 50 times more active than the free drug. Control experiments with other constructs (e.g., allopurinol riboside linked to the mannose-free polymer) confirmed that the enhancement of activity was indeed achieved by means of the mannose homing device.
Full text
PDF
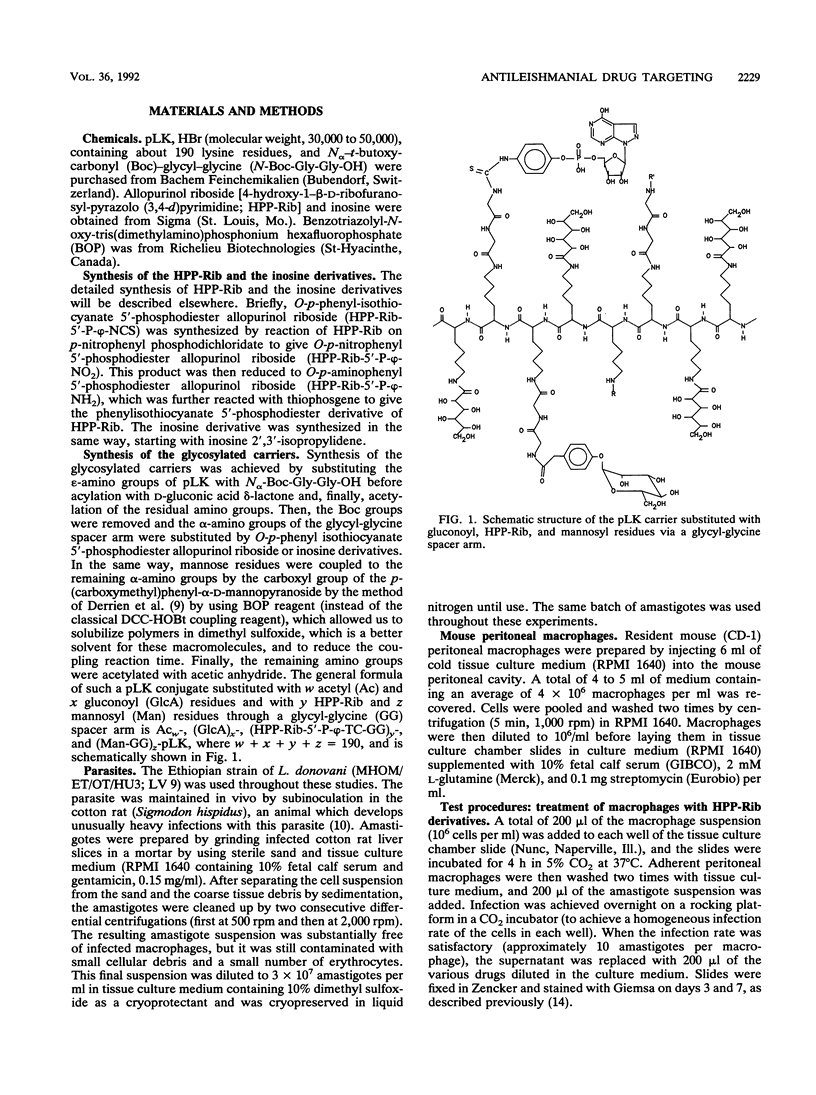
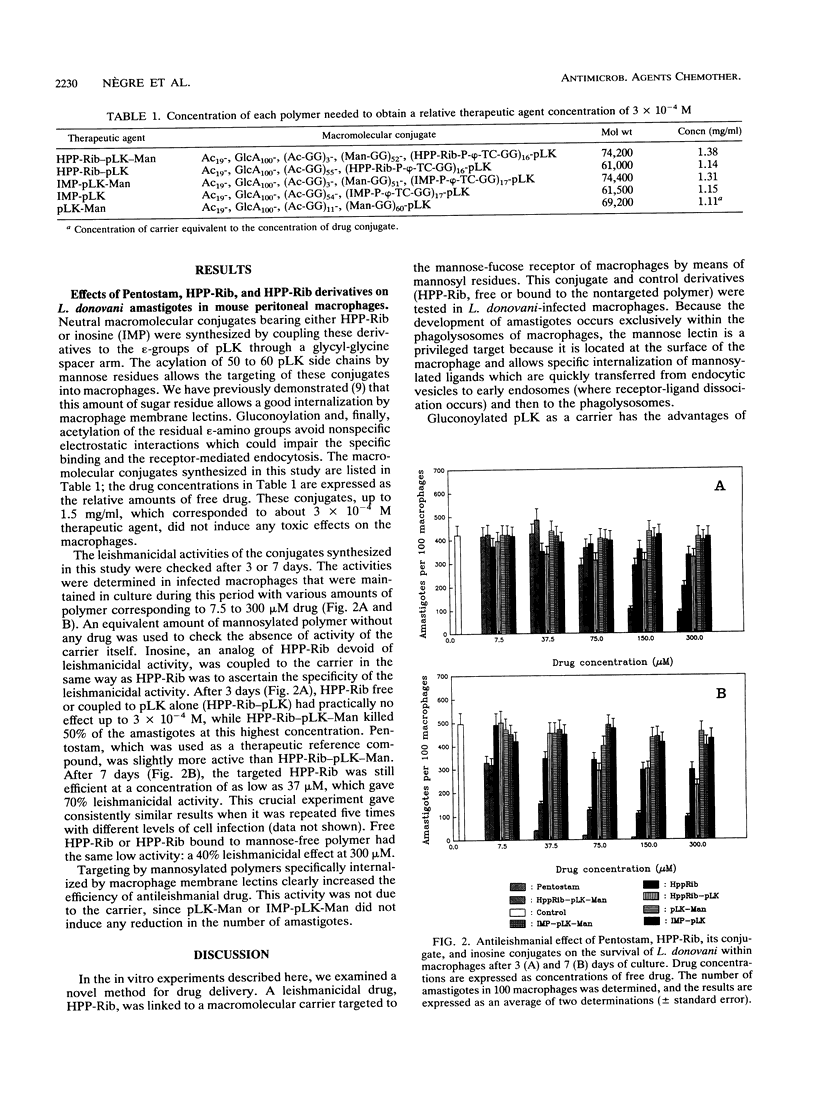


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berens R. L., Marr J. J., Nelson D. J., LaFon S. W. Antileishmanial effect of allopurinol and allopurinol ribonucleoside on intracellular forms of Leishmania donovani. Biochem Pharmacol. 1980 Sep 1;29(17):2397–2398. doi: 10.1016/0006-2952(80)90275-0. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Hanson W. L., Chapman W. L., Alving C. R., Lopez-Berestein G. Antileishmanial activity of liposome-encapsulated amphotericin B in hamsters and monkeys. Antimicrob Agents Chemother. 1986 Dec;30(6):847–851. doi: 10.1128/aac.30.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Waddell D., Hanson B. D. Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate. Antimicrob Agents Chemother. 1985 Jun;27(6):916–920. doi: 10.1128/aac.27.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Webster H. K. In vitro effects of mycophenolic acid and allopurinol against Leishmania tropica in human macrophages. Antimicrob Agents Chemother. 1982 Jun;21(6):887–891. doi: 10.1128/aac.21.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. D., Watson G. J., Ward R. J. The use of Pentostam liposomes in the chemotherapy of experimental leishmaniasis. Trans R Soc Trop Med Hyg. 1977;71(6):550–552. doi: 10.1016/0035-9203(77)90155-9. [DOI] [PubMed] [Google Scholar]
- Chakraborty P., Bhaduri A. N., Das P. K. Neoglycoproteins as carriers for receptor-mediated drug targeting in the treatment of experimental visceral leishmaniasis. J Protozool. 1990 Sep-Oct;37(5):358–364. doi: 10.1111/j.1550-7408.1990.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri G., Mukhopadhyay A., Basu S. K. Selective delivery of drugs to macrophages through a highly specific receptor. An efficient chemotherapeutic approach against leishmaniasis. Biochem Pharmacol. 1989 Sep 15;38(18):2995–3002. doi: 10.1016/0006-2952(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Chunge C. N., Gachihi G., Muigai R., Wasunna K., Rashid J. R., Chulay J. D., Anabwani G., Oster C. N., Bryceson A. D. Visceral leishmaniasis unresponsive to antimonial drugs. III. Successful treatment using a combination of sodium stibogluconate plus allopurinol. Trans R Soc Trop Med Hyg. 1985;79(5):715–718. doi: 10.1016/0035-9203(85)90200-7. [DOI] [PubMed] [Google Scholar]
- Derrien D., Midoux P., Petit C., Nègre E., Mayer R., Monsigny M., Roche A. C. Muramyl dipeptide bound to poly-L-lysine substituted with mannose and gluconoyl residues as macrophage activators. Glycoconj J. 1989;6(2):241–255. doi: 10.1007/BF01050652. [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Dolan T. F., Coombs G. H., Baillie A. J. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol. 1988 Mar;40(3):161–165. doi: 10.1111/j.2042-7158.1988.tb05210.x. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Berens R. L. Antileishmanial effect of allopurinol. II. Relationship of adenine metabolism in Leishmania species to the action of allopurinol. J Infect Dis. 1977 Dec;136(6):724–732. doi: 10.1093/infdis/136.6.724. [DOI] [PubMed] [Google Scholar]
- Mattock N. M., Peters W. The experimental chemotherapy of leishmaniasis. I: Techniques for the study of drug action in tissue culture. Ann Trop Med Parasitol. 1975 Sep;69(3):349–357. [PubMed] [Google Scholar]
- Midoux P., Negre E., Roche A. C., Mayer R., Monsigny M., Balzarini J., De Clercq E., Mayer E., Ghaffar A., Gangemi J. D. Drug targeting: anti-HSV-1 activity of mannosylated polymer-bound 9-(2-phosphonylmethoxyethyl)adenine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1044–1049. doi: 10.1016/0006-291x(90)90628-z. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Bailly P. Tumoricidal activation of murine alveolar macrophages by muramyldipeptide substituted mannosylated serum albumin. Biochem Biophys Res Commun. 1984 Jun 15;121(2):579–584. doi: 10.1016/0006-291x(84)90221-3. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Midoux P. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol Cell. 1984;51(2):187–196. doi: 10.1111/j.1768-322x.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A., Chaudhuri G., Arora S. K., Sehgal S., Basu S. K. Receptor-mediated drug delivery to macrophages in chemotherapy of leishmaniasis. Science. 1989 May 12;244(4905):705–707. doi: 10.1126/science.2717947. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Bugge C. J., Elion G. B., Berens R. L., Marr J. J. Metabolism of pyrazolo(3,4-d)pyrimidines in Leishmania braziliensis and Leishmania donovani. Allopurinol, oxipurinol, and 4-aminopyrazolo(3,4-d)pyrimidine. J Biol Chem. 1979 May 25;254(10):3959–3964. [PubMed] [Google Scholar]
- New R. R., Chance M. L., Thomas S. C., Peters W. Antileishmanial activity of antimonials entrapped in liposomes. Nature. 1978 Mar 2;272(5648):55–56. doi: 10.1038/272055a0. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Marr J. J. Antileishmanial effect of allopurinol. Antimicrob Agents Chemother. 1974 May;5(5):469–472. doi: 10.1128/aac.5.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A. C., Bailly P., Monsigny M. Macrophage activation by MDP bound to neoglycoproteins: metastasis eradication in mice. Invasion Metastasis. 1985;5(4):218–232. [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]



