Abstract
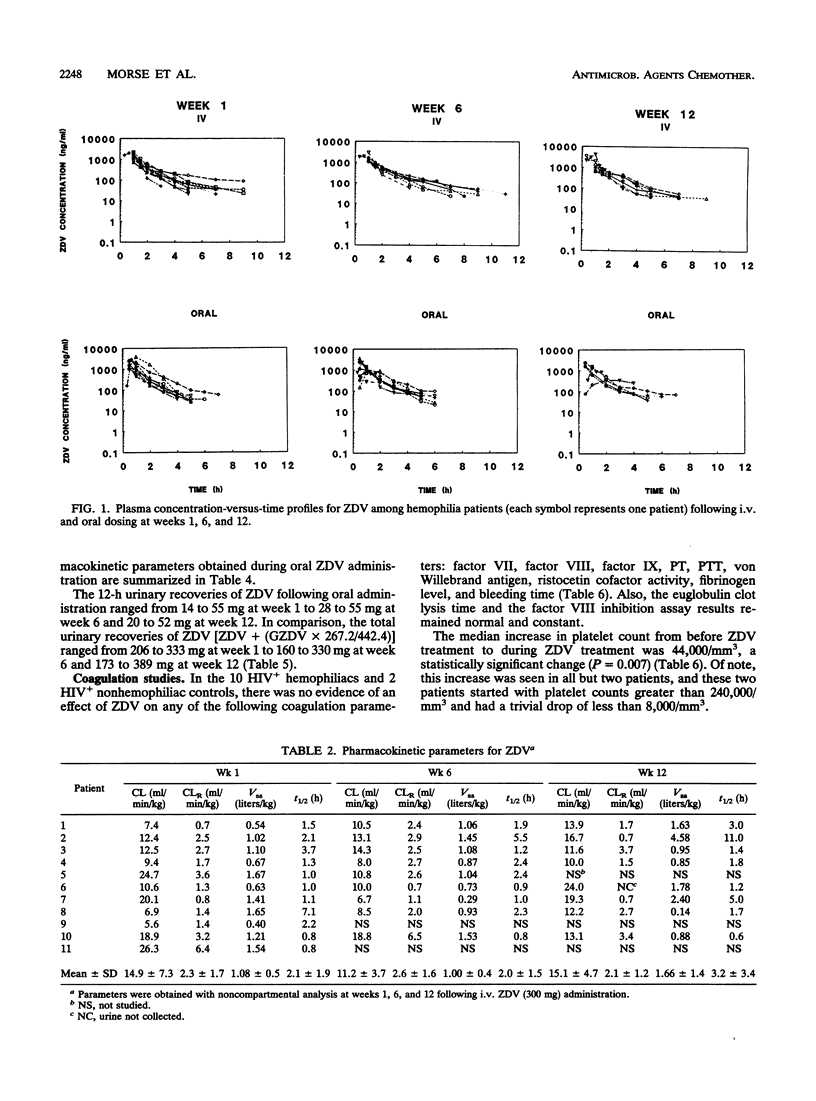
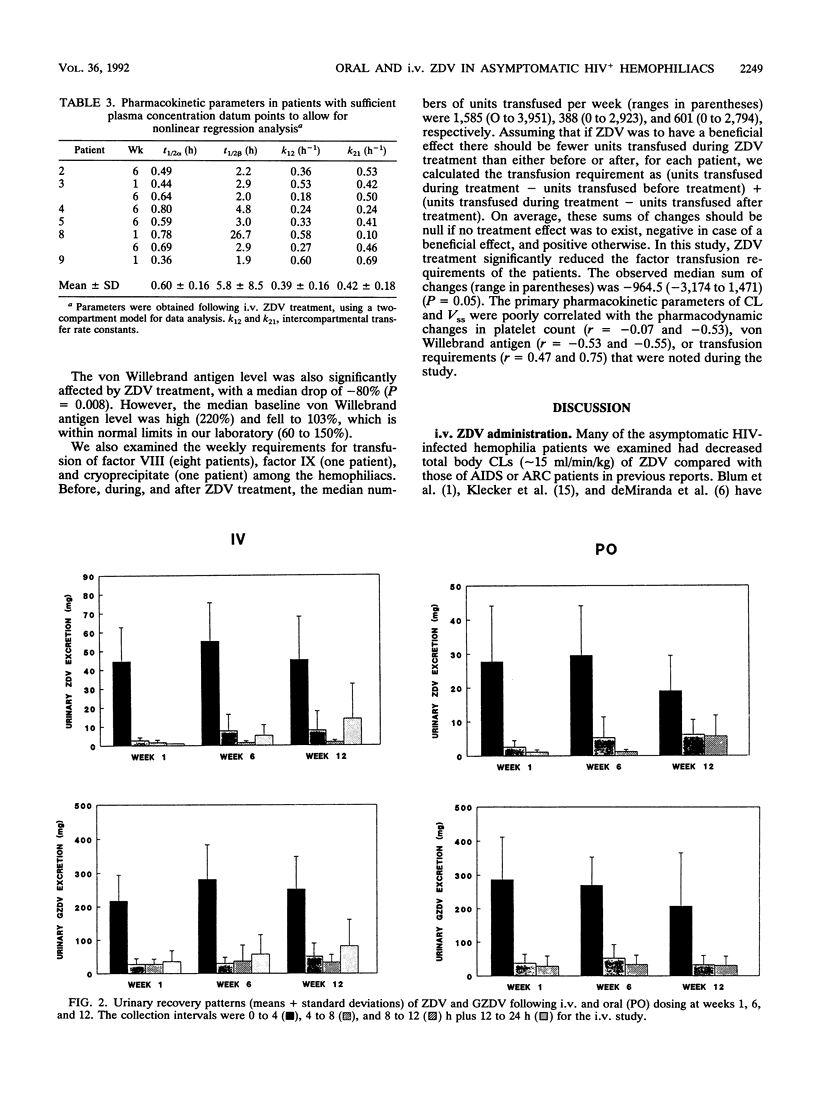
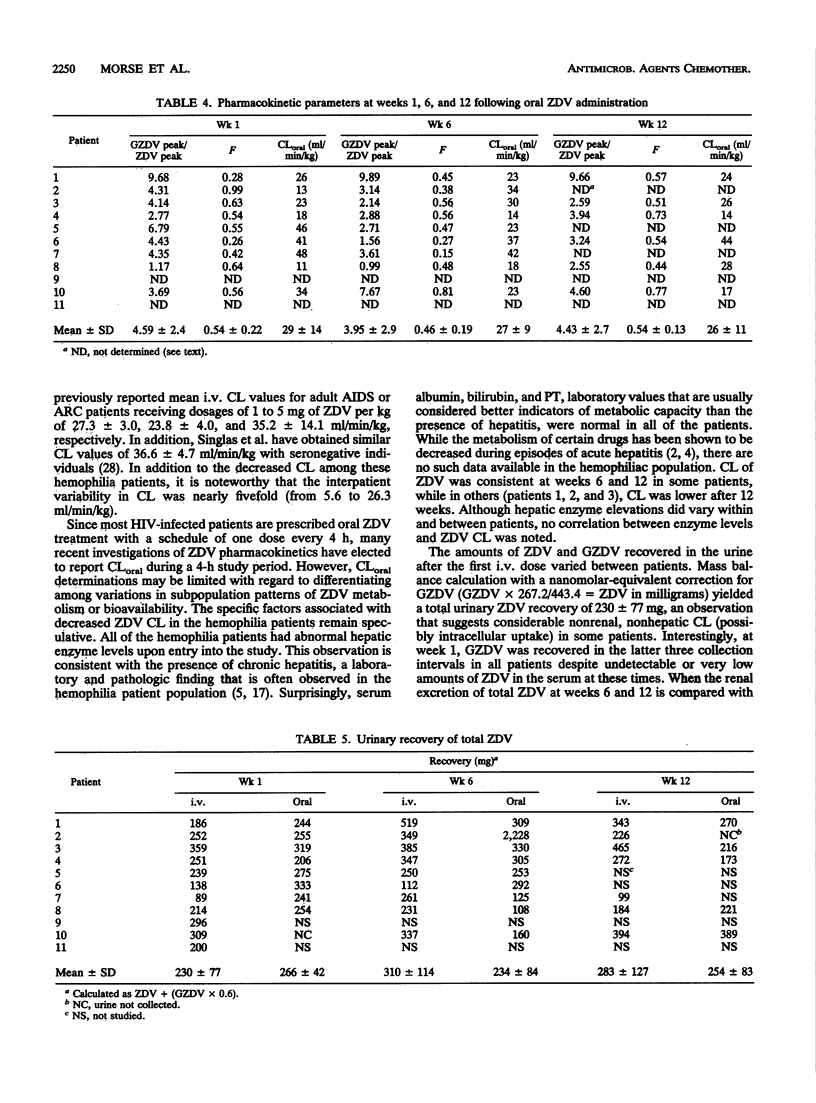
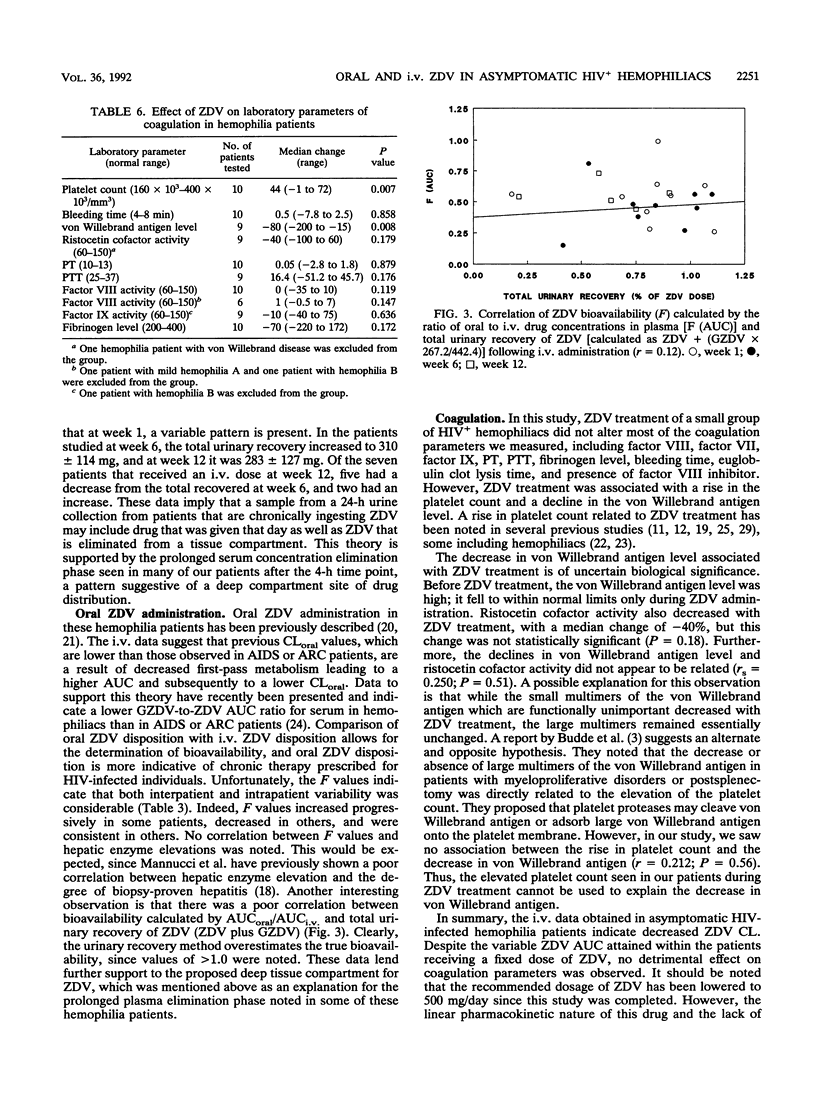
Pharmacokinetic and coagulation studies were carried out over a 12-week period with 11 asymptomatic hemophilia patients with human immunodeficiency virus infection receiving zidovudine (ZDV). The patients received 300 mg every 4 h while awake (the accepted dose at the time of this study); consecutive 24-h intravenous (i.v.) and 12-h oral pharmacokinetic studies were conducted at weeks 1, 6, and 12. Coagulation studies were conducted at weeks 0, 4, 8, and 12. The numbers of units of factors VIII and IX and cryoprecipitate transfused during the 12-week periods before, during, and after ZDV treatment were recorded. Following i.v. and oral ZDV administration, the concentration in plasma declined rapidly over the first 4 h, and in some patients, ZDV was still detectable at 4 to 10 h. The i.v. total clearances (means +/- standard deviations) were 14.9 +/- 7.3, 11.2 +/- 3.7, and 15.1 +/- 4.7 ml/min/kg of body weight. The i.v. distribution volumes were 1.08 +/- 0.5, 1.0 +/- 0.4, and 1.65 +/- 1.4 liters/kg. The bioavailabilities were 0.54 +/- 0.22, 0.46 +/- 0.19, and 0.59 +/- 0.13 at weeks 1, 6, and 12, respectively. The pattern of ZDV-glucuronide (GZDV) disposition was similar to that of ZDV, and the peak plasma GZDV-to-ZDV ratio was higher after oral dosing, consistent with first-pass metabolism. In some individuals, up to 33% of an i.v. dose was excreted unchanged. At weeks 6 and 12, greater than 300 mg of total ZDV (GZDV plus ZDV) was recovered in the urine of some patients, suggesting tissue redistribution. Concentration in plasma after oral ZDV administration were variable, both within and between patients. The von Willebrand antigen level consistently decreased throughout the study but was not accompanied by a parallel change in ristocetin cofactor A activity, and no clinical adverse effects on coagulation were noted. This study demonstrates that ZDV can be used in hemophilia patients without worsening of their bleeding tendencies. The clinical significance of decreased ZDV clearance and the prolonged terminal elimination phase of ZDV will require further study with patients receiving chronic ZDV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum M. R., Liao S. H., Good S. S., de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988 Aug 29;85(2A):189–194. [PubMed] [Google Scholar]
- Breimer D. D., Zilly W., Richter E. Pharmacokinetics of hexobarbital in acute hepatitis and after apparent recovery. Clin Pharmacol Ther. 1975 Oct;18(4):433–440. doi: 10.1002/cpt1975184433. [DOI] [PubMed] [Google Scholar]
- Burnett D. A., Barak A. J., Tuma D. J., Sorrell M. F. Altered elimination of antipyrine in patients with acute viral hepatitis. Gut. 1976 May;17(5):341–344. doi: 10.1136/gut.17.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum A. I., Blatt P. M., Levine P. H. Abnormal serum transaminase levels in patients with hemophilia A. Arch Intern Med. 1982 Mar;142(3):481–484. [PubMed] [Google Scholar]
- Evatt B. L., Ramsey R. B., Lawrence D. N., Zyla L. D., Curran J. W. The acquired immunodeficiency syndrome in patients with hemophilia. Ann Intern Med. 1984 Apr;100(4):499–504. doi: 10.7326/0003-4819-100-4-499. [DOI] [PubMed] [Google Scholar]
- Eyster M. E., Ballard J. O., Gail M. H., Drummond J. E., Goedert J. J. Predictive markers for the acquired immunodeficiency syndrome (AIDS) in hemophiliacs: persistence of p24 antigen and low T4 cell count. Ann Intern Med. 1989 Jun 15;110(12):963–969. doi: 10.7326/0003-4819-110-12-963. [DOI] [PubMed] [Google Scholar]
- Goedert J. J., Kessler C. M., Aledort L. M., Biggar R. J., Andes W. A., White G. C., 2nd, Drummond J. E., Vaidya K., Mann D. L., Eyster M. E. A prospective study of human immunodeficiency virus type 1 infection and the development of AIDS in subjects with hemophilia. N Engl J Med. 1989 Oct 26;321(17):1141–1148. doi: 10.1056/NEJM198910263211701. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. S., Wolfe P. R., Chafey S. Response of AIDS-related thrombocytopenia to intravenous and oral azidothymidine (3'-azido-3'-deoxythymidine). AIDS Res Hum Retroviruses. 1987 Summer;3(2):109–114. doi: 10.1089/aid.1987.3.109. [DOI] [PubMed] [Google Scholar]
- Hymes K. B., Greene J. B., Karpatkin S. The effect of azidothymidine on HIV-related thrombocytopenia. N Engl J Med. 1988 Feb 25;318(8):516–517. doi: 10.1056/NEJM198802253180812. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Lawrence D. N., Evatt B. L., Bregman D. J., Zyla L. D., Curran J. W., Aledort L. M., Eyster M. E., Brownstein A. P., Carman C. J. Acquired immunodeficiency syndrome among patients attending hemophilia treatment centers and mortality experience of hemophiliacs in the United States. Am J Epidemiol. 1985 Jun;121(6):797–810. doi: 10.1093/oxfordjournals.aje.a114051. [DOI] [PubMed] [Google Scholar]
- Jones P. Zidovudine: experience at the Newcastle Haemophilia Centre. J Infect. 1989 Jan;18 (Suppl 1):53–58. doi: 10.1016/s0163-4453(89)80080-5. [DOI] [PubMed] [Google Scholar]
- Klecker R. W., Jr, Collins J. M., Yarchoan R., Thomas R., Jenkins J. F., Broder S., Myers C. E. Plasma and cerebrospinal fluid pharmacokinetics of 3'-azido-3'-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987 Apr;41(4):407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- Levine P. H., McVerry B. A., Attock B., Dormandy K. M. Health of the intensively treated hemophiliac, with special reference to abnormal liver chemistries and splenomegaly. Blood. 1977 Jul;50(1):1–9. [PubMed] [Google Scholar]
- Levine P. H. The acquired immunodeficiency syndrome in persons with hemophilia. Ann Intern Med. 1985 Nov;103(5):723–726. doi: 10.7326/0003-4819-103-5-723. [DOI] [PubMed] [Google Scholar]
- Mannucci P. M., Capitanio A., Del Ninno E., Colombo M., Pareti F., Ruggeri Z. M. Asymptomatic liver disease in haemophiliacs. J Clin Pathol. 1975 Aug;28(8):620–624. doi: 10.1136/jcp.28.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J. S., Le T., Fanning M., Gelmon K., Tsoukas C., Falutz J., O'Shaughnessy M., Wainberg M. A., Ruedy J. The effect of zidovudine on platelet count in HIV-infected individuals. J Acquir Immune Defic Syndr. 1990;3(6):565–570. [PubMed] [Google Scholar]
- Morse G. D., Olson J., Portmore A., Taylor C., Plank C., Reichman R. C. Pharmacokinetics of orally administered zidovudine among patients with hemophilia and asymptomatic human immunodeficiency virus (HIV) infection. Antiviral Res. 1989 Mar;11(2):57–65. doi: 10.1016/0166-3542(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Morse G. D., Portmore A., Olson J., Taylor C., Plank C., Reichman R. C. Multiple-dose pharmacokinetics of oral zidovudine in hemophilia patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1990 Mar;34(3):394–397. doi: 10.1128/aac.34.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzer S., Stain C., Benda H., Mannhalter C. Effects of 3-azidothymidine on platelet counts, indium-111-labelled platelet kinetics, and antiplatelet antibodies. Vox Sang. 1989;57(2):120–126. doi: 10.1111/j.1423-0410.1989.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Fischl M. A., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Hirsch M. S. The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):192–197. doi: 10.1056/NEJM198707233170402. [DOI] [PubMed] [Google Scholar]
- Rocci M. L., Jr, Jusko W. J. LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed. 1983 Jun;16(3):203–216. doi: 10.1016/0010-468x(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Schimpf K., Brackmann H. H., Kreuz W., Kraus B., Haschke F., Schramm W., Moesseler J., Auerswald G., Sutor A. H., Koehler K. Absence of anti-human immunodeficiency virus types 1 and 2 seroconversion after the treatment of hemophilia A or von Willebrand's disease with pasteurized factor VIII concentrate. N Engl J Med. 1989 Oct 26;321(17):1148–1152. doi: 10.1056/NEJM198910263211702. [DOI] [PubMed] [Google Scholar]
- Singlas E., Pioger J. C., Taburet A. M., Colin J. N., Fillastre J. P. Zidovudine disposition in patients with severe renal impairment: influence of hemodialysis. Clin Pharmacol Ther. 1989 Aug;46(2):190–197. doi: 10.1038/clpt.1989.125. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Good S. S., Yarchoan R., Thomas R. V., Blum M. R., Myers C. E., Broder S. Alteration of zidovudine pharmacokinetics by probenecid in patients with AIDS or AIDS-related complex. Clin Pharmacol Ther. 1989 Nov;46(5):494–500. doi: 10.1038/clpt.1989.176. [DOI] [PubMed] [Google Scholar]




