Abstract
The genetic determinant encoding gentamicin resistance (Gmr) on the beta-lactamase encoding plasmid pBEM10 of Enterococcus faecalis HH22 is carried on a transposon, termed Tn5281, that is highly related to the staphylococcal Gmr transposons Tn4001 found in Australian isolates of Staphylococcus aureus and Tn4031 found in United States isolates of Staphylococcus epidermidis. We have now studied plasmid DNA from Gmr strains of E. faecalis isolated from diverse geographical locations (Houston, Pennsylvania, Thailand, and Chile) by using restriction endonuclease analysis and DNA-DNA hybridization to determine whether other Gmr E. faecalis carry Tn5281 or a similar type of element. We also compared these enterococci to several United States isolates of Staphylococcus aureus with nonmobile Gmr determinants. Three E. faecalis isolates (from Houston and Chile) carried Tn5281-like elements, whereas two isolates (from Houston and Pennsylvania) had restriction endonuclease and DNA-DNA hybridization patterns more similar to those of the Tn4001-IS257 hybrid found in the nonmobile Gmr determinants in United States isolates of S. aureus. A strain from Thailand had a third pattern unrelated to either Tn5281 or the nonmobile Gmr determinants present in United States isolates of S. aureus. Our results demonstrate that there is both similarity and diversity between the Gmr determinant of strains of E. faecalis isolated in diverse geographic locations.
Full text
PDF
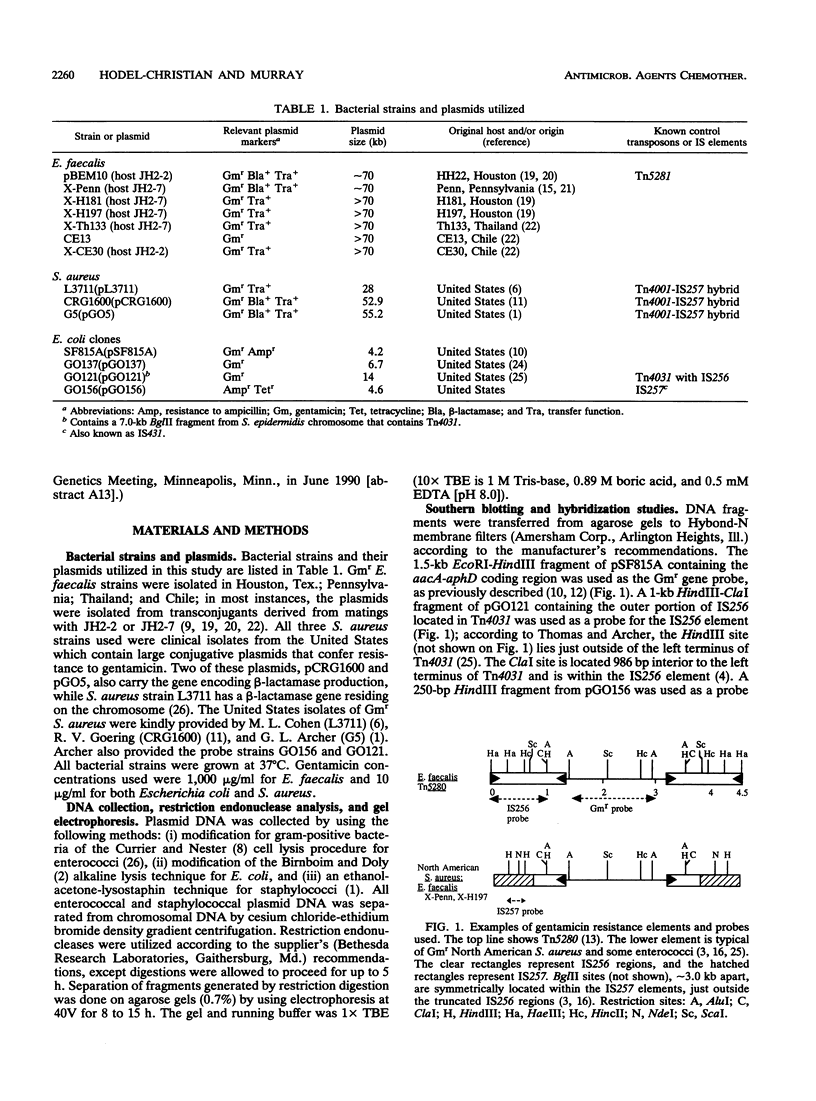

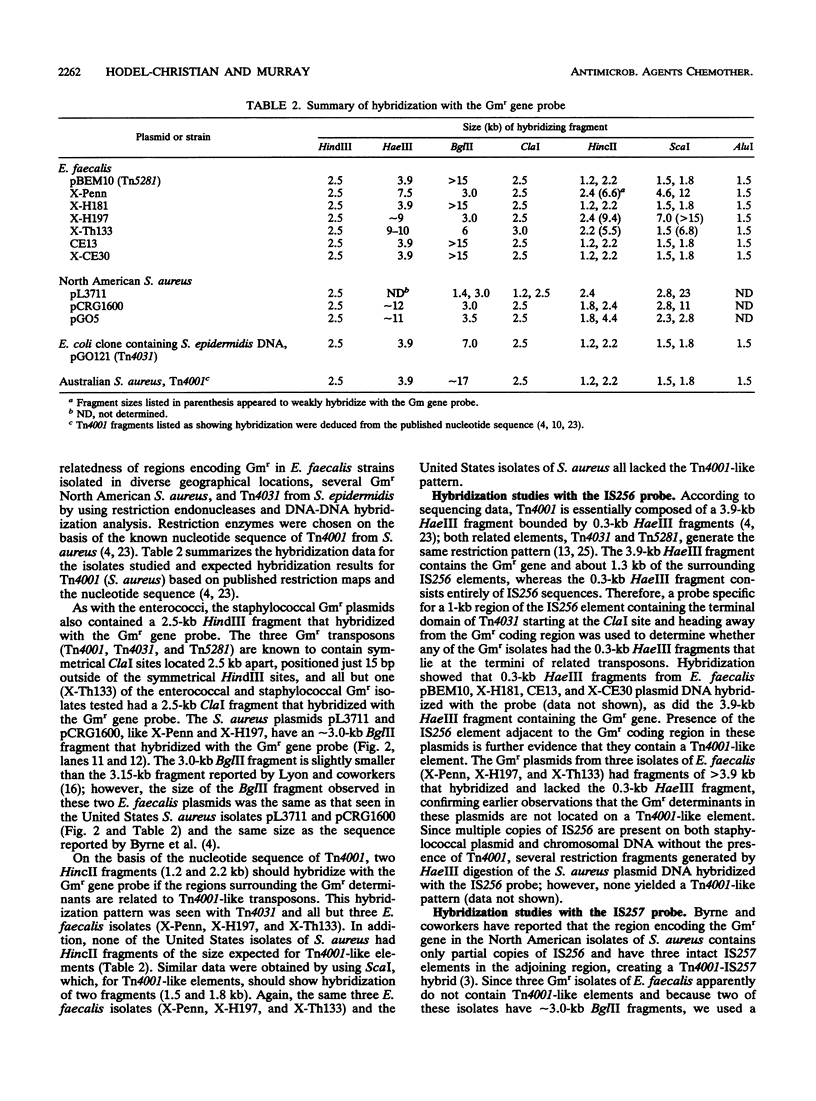
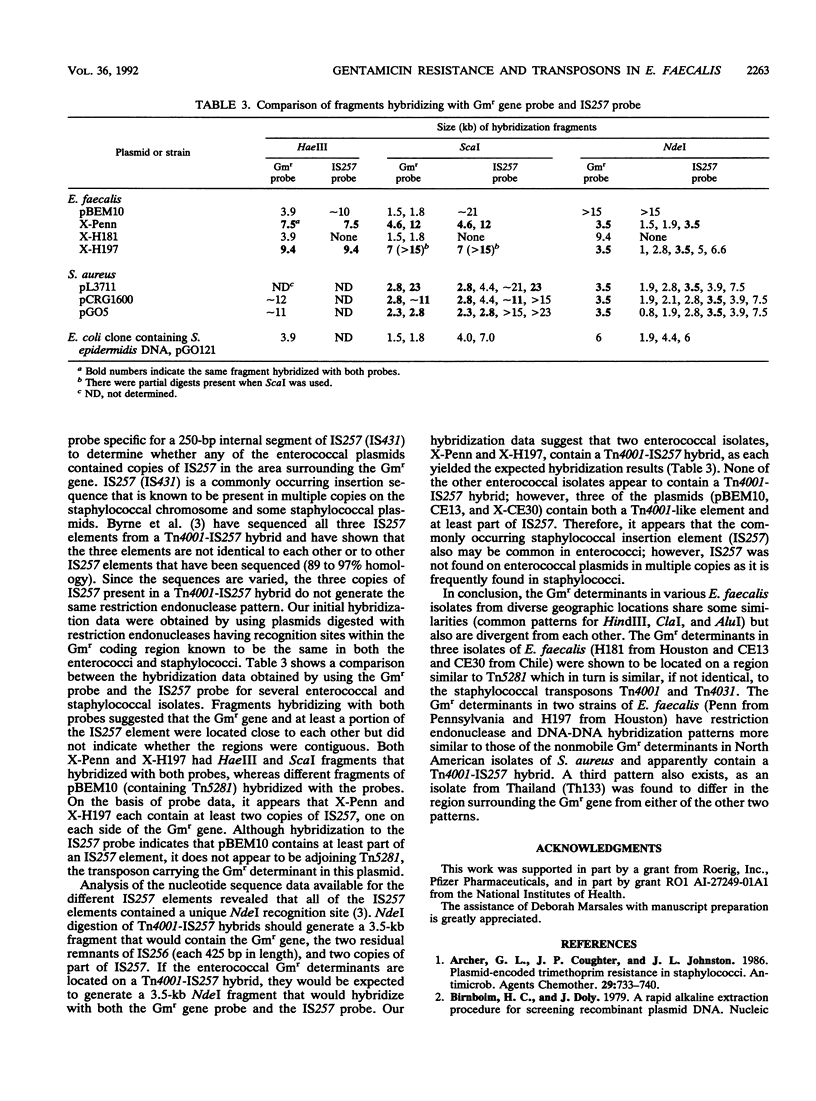

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Coughter J. P., Johnston J. L. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 1986 May;29(5):733–740. doi: 10.1128/aac.29.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. E., Gillespie M. T., Skurray R. A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990 Nov;34(11):2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Wong E. S., Falkow S. Common R-plasmids in Staphylococcus aureus and Staphylococcus epidermidis during a nosocomial Staphylococcus aureus outbreak. Antimicrob Agents Chemother. 1982 Feb;21(2):210–215. doi: 10.1128/aac.21.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Dunny G., Yuhasz M., Ehrenfeld E. Genetic and physiological analysis of conjugation in Streptococcus faecalis. J Bacteriol. 1982 Aug;151(2):855–859. doi: 10.1128/jb.151.2.855-859.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991 Jun;35(6):1147–1152. doi: 10.1128/aac.35.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Mobilization of the gentamicin resistance gene in Enterococcus faecalis. Antimicrob Agents Chemother. 1990 Jun;34(6):1278–1280. doi: 10.1128/aac.34.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman M., Pitsakis P. G., Rosenberg A., Hessen M. T., Abrutyn E., Murray B. E., Levison M. E. beta-Lactamase production in experimental endocarditis due to aminoglycoside-resistant Streptococcus faecalis. J Infect Dis. 1987 Jun;155(6):1226–1232. doi: 10.1093/infdis/155.6.1226. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Byrne M. E., May J. W., Skurray R. A. Plasmid-mediated resistance to gentamicin in Staphylococcus aureus: the involvement of a transposon. J Med Microbiol. 1987 Mar;23(2):101–110. doi: 10.1099/00222615-23-2-101. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Tn4001: a gentamicin and kanamycin resistance transposon in Staphylococcus aureus. Mol Gen Genet. 1984;193(3):554–556. doi: 10.1007/BF00382099. [DOI] [PubMed] [Google Scholar]
- Martel A., Masson M., Moreau N., Le Goffic F. Kinetic studies of aminoglycoside acetyltransferase and phosphotransferase from Staphylococcus aureus RPAL. Relationship between the two activities. Eur J Biochem. 1983 Jul 1;133(3):515–521. doi: 10.1111/j.1432-1033.1983.tb07494.x. [DOI] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Murray B. E., An F. Y., Clewell D. B. Plasmids and pheromone response of the beta-lactamase producer Streptococcus (Enterococcus) faecalis HH22. Antimicrob Agents Chemother. 1988 Apr;32(4):547–551. doi: 10.1128/aac.32.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Church D. A., Wanger A., Zscheck K., Levison M. E., Ingerman M. J., Abrutyn E., Mederski-Samoraj B. Comparison of two beta-lactamase-producing strains of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Dec;30(6):861–864. doi: 10.1128/aac.30.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch D. A., Byrne M. E., Kong Y. C., Skurray R. A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J Gen Microbiol. 1987 Nov;133(11):3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989 Feb;171(2):684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Mobility of gentamicin resistance genes from staphylococci isolated in the United States: identification of Tn4031, a gentamicin resistance transposon from Staphylococcus epidermidis. Antimicrob Agents Chemother. 1989 Aug;33(8):1335–1341. doi: 10.1128/aac.33.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E. Comparison of enterococcal and staphylococcal beta-lactamase plasmids. J Infect Dis. 1990 Jan;161(1):54–58. doi: 10.1093/infdis/161.1.54. [DOI] [PubMed] [Google Scholar]




