Abstract
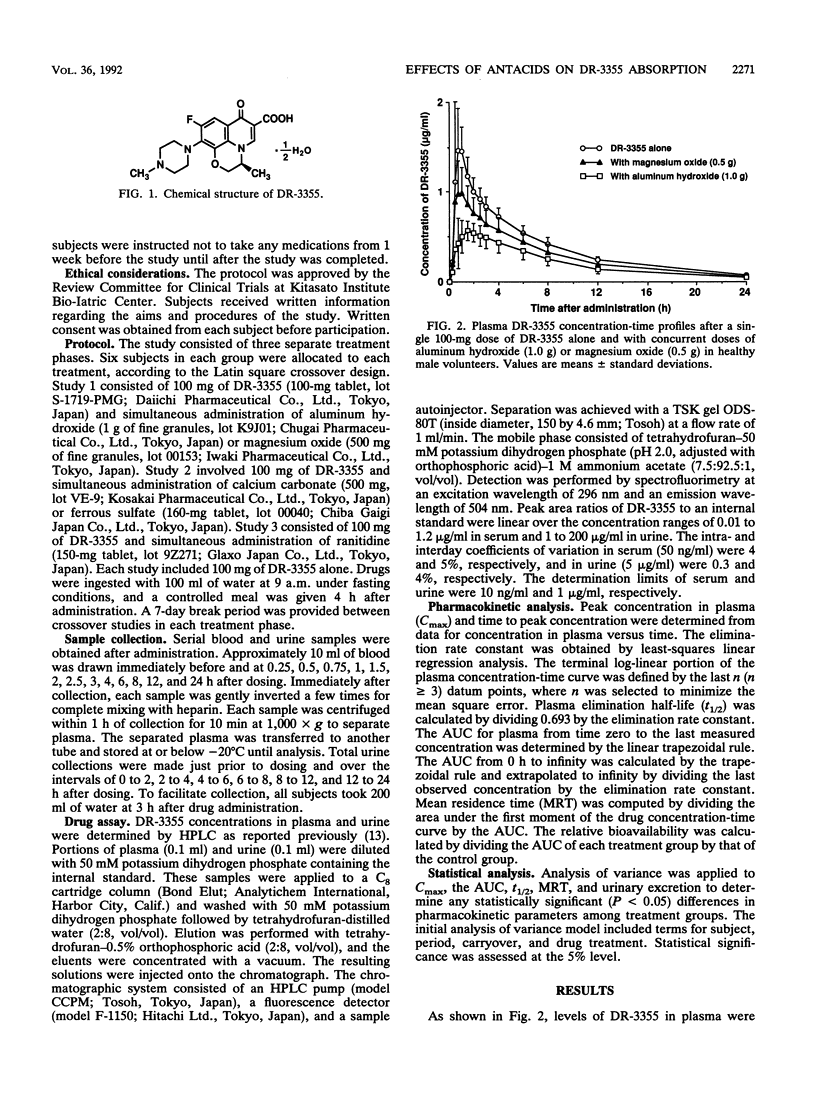
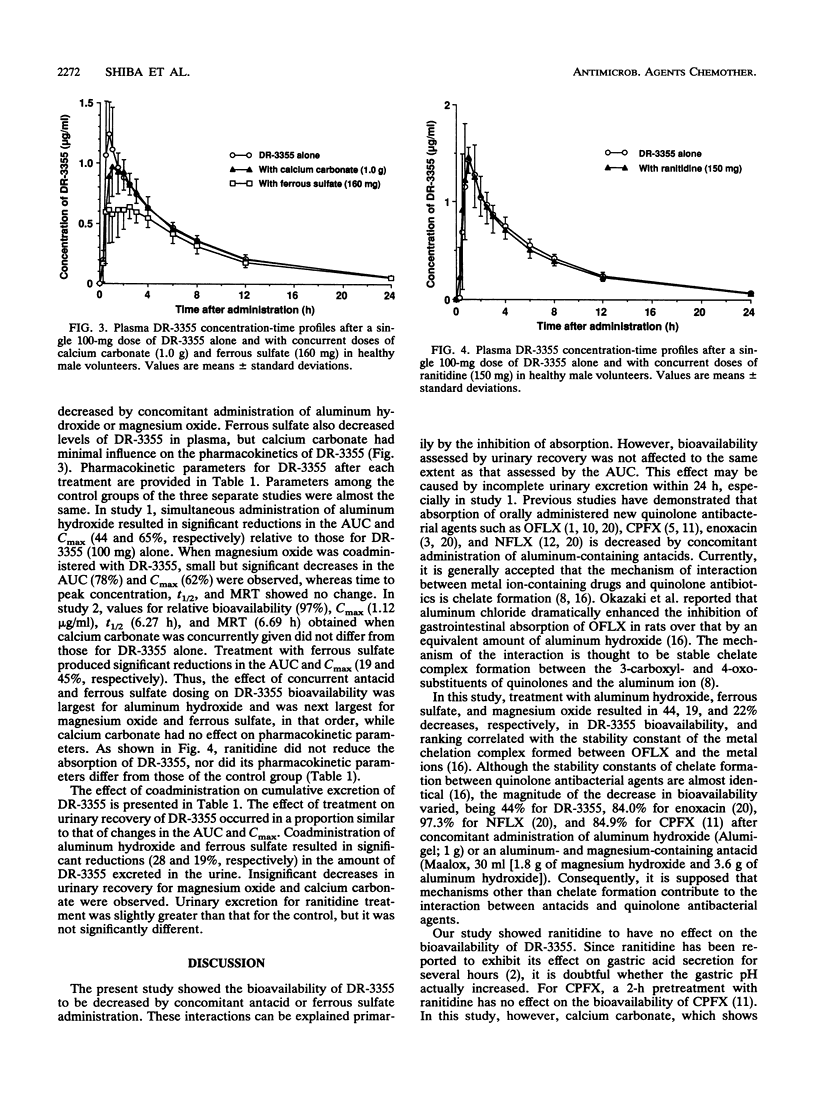
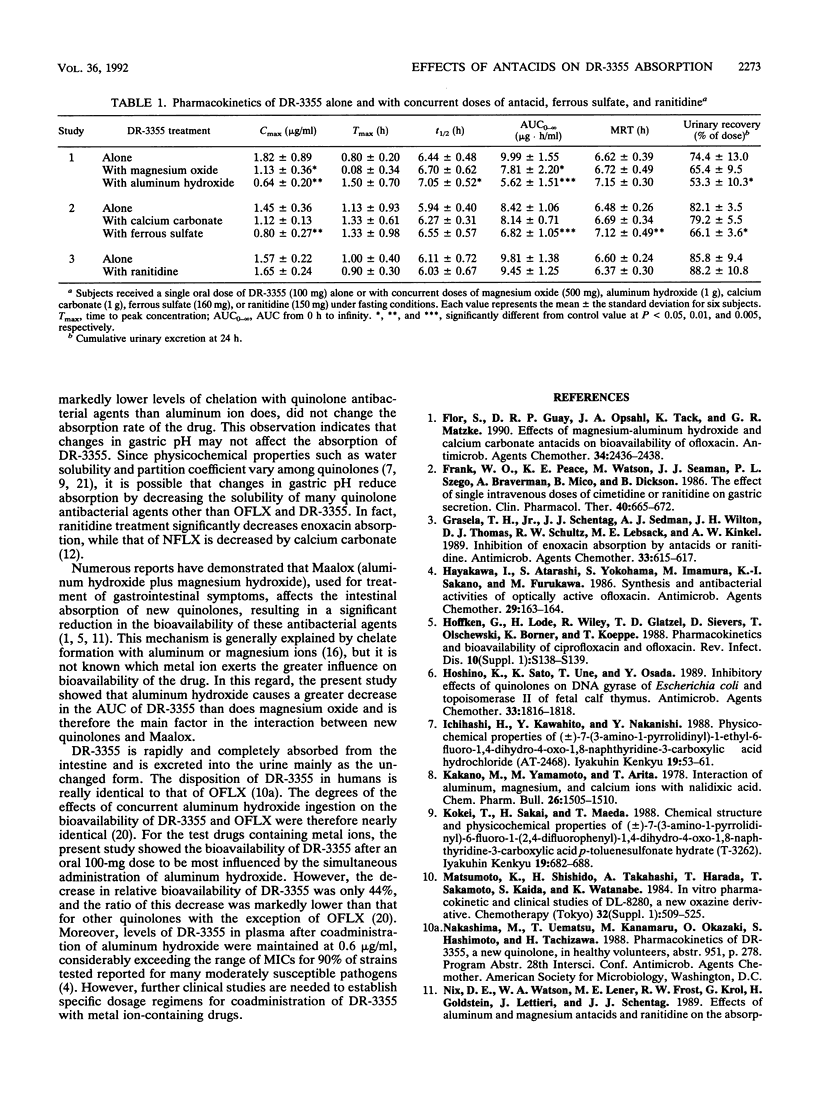
This study examined the effects of widely used antacids (aluminum hydroxide, magnesium oxide, and calcium carbonate), ferrous sulfate, and ranitidine on the absorption of a fluorinated quinolone, (-)-(S)-9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro- 7H- pyrido-[1,2,3,-de][1,4]benzoxazine-6-carboxylic acid hemihydrate (DR-3355), in healthy male volunteers enrolled in three separate randomized crossover studies. Study 1 used 100-mg doses of DR-3355 and concurrent doses of aluminum hydroxide (1 g) or magnesium oxide (500 mg), while study 2 used DR-3355 (100 mg) and concurrent ferrous sulfate (160 mg) or calcium carbonate (1 g). Study 3 used DR-3355 (100 mg) and concurrent ranitidine (150 mg). Each study included control doses of DR-3355 (100 mg) alone. When aluminum hydroxide, ferrous sulfate, and magnesium oxide were coadministered with DR-3355, the relative bioavailability of DR-3355 was decreased to 56, 81, and 78%, respectively, of that for DR-3355 (100 mg) alone. Urinary excretion of DR-3355 was also significantly decreased by coadministration of these drugs. Thus, the magnitude of the decrease in the area under the concentration-time curve for DR-3355 varied among antacids, and the ranking of their inhibitory effects correlated with previously reported rankings of stability constants for chelate formation. DR-3355 bioavailability was not influenced by the concurrent administration of calcium carbonate and ranitidine, indicating that changes in gastric pH do not affect DR-3355 absorption.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flor S., Guay D. R., Opsahl J. A., Tack K., Matzke G. R. Effects of magnesium-aluminum hydroxide and calcium carbonate antacids on bioavailability of ofloxacin. Antimicrob Agents Chemother. 1990 Dec;34(12):2436–2438. doi: 10.1128/aac.34.12.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W. O., Peace K. E., Watson M., Seaman J. J., Szego P. L., Braverman A., Mico B., Dickson B. The effect of single intravenous doses of cimetidine or ranitidine on gastric secretion. Clin Pharmacol Ther. 1986 Dec;40(6):665–672. doi: 10.1038/clpt.1986.242. [DOI] [PubMed] [Google Scholar]
- Grasela T. H., Jr, Schentag J. J., Sedman A. J., Wilton J. H., Thomas D. J., Schultz R. W., Lebsack M. E., Kinkel A. W. Inhibition of enoxacin absorption by antacids or ranitidine. Antimicrob Agents Chemother. 1989 May;33(5):615–617. doi: 10.1128/aac.33.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa I., Atarashi S., Yokohama S., Imamura M., Sakano K., Furukawa M. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother. 1986 Jan;29(1):163–164. doi: 10.1128/aac.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Sato K., Une T., Osada Y. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother. 1989 Oct;33(10):1816–1818. doi: 10.1128/aac.33.10.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Yamamoto M., Arita T. Interactions of aluminum, magnesium, and calcium ions with nalidixic acid. Chem Pharm Bull (Tokyo) 1978 May;26(5):1505–1510. doi: 10.1248/cpb.26.1505. [DOI] [PubMed] [Google Scholar]
- Nix D. E., Wilton J. H., Ronald B., Distlerath L., Williams V. C., Norman A. Inhibition of norfloxacin absorption by antacids. Antimicrob Agents Chemother. 1990 Mar;34(3):432–435. doi: 10.1128/aac.34.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki O., Aoki H., Hakusui H. High-performance liquid chromatographic determination of (S)-(-)-ofloxacin and its metabolites in serum and urine using a solid-phase clean-up. J Chromatogr. 1991 Feb 15;563(2):313–322. doi: 10.1016/0378-4347(91)80037-d. [DOI] [PubMed] [Google Scholar]
- Okazaki O., Kojima C., Hakusui H., Nakashima M. Enantioselective disposition of ofloxacin in humans. Antimicrob Agents Chemother. 1991 Oct;35(10):2106–2109. doi: 10.1128/aac.35.10.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki O., Kurata T., Hakusui H., Tachizawa H. Stereoselective glucuronidation of ofloxacin in rat liver microsomes. Drug Metab Dispos. 1991 Mar-Apr;19(2):376–380. [PubMed] [Google Scholar]
- Okazaki O., Kurata T., Tachizawa H. Stereoselective metabolic disposition of enantiomers of ofloxacin in rats. Xenobiotica. 1989 Apr;19(4):419–429. doi: 10.3109/00498258909042283. [DOI] [PubMed] [Google Scholar]
- Polk R. E., Healy D. P., Sahai J., Drwal L., Racht E. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1989 Nov;33(11):1841–1844. doi: 10.1128/aac.33.11.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]


