Abstract
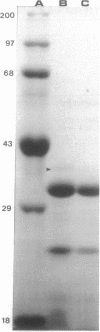
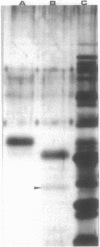
The most common mechanism of antibiotic resistance in multiply resistant Pseudomonas cepacia is decreased porin-mediated outer membrane permeability. In some gram-negative organisms this form of antibiotic resistance can be induced by growth in the presence of weak acids, such as salicylates, which suppress porin synthesis. To determine the effects of salicylates on outer membrane permeability of P. cepacia, a susceptible laboratory strain, 249-2, was grown in 10 mM sodium salicylate. Antibiotic susceptibility and uptake, as well as outer membrane protein patterns, were compared between strain 249-2 grown with and without salicylates. The MICs of chloramphenicol, trimethoprim, ciprofloxacin, and ceftazidime were compared between organisms grown in standard and salicylate-containing medium and are as follows: chloramphenicol, 12.5 versus 100 micrograms/ml; trimethoprim, 0.78 versus 3.125 micrograms/ml; ciprofloxacin, 0.4 versus 1.56 micrograms/ml; ceftazidime, 3.125 versus 3.125 micrograms/ml. The permeability of beta-lactam antibiotics was calculated from the rate of hydrolysis of the chromogenic cephalosporin, PADAC. There was no significant difference between strains grown in the presence and absence of salicylate. By using high-pressure liquid chromatography quantitation of loss from culture medium, the effect of 10 mM salicylate on the cellular permeability of chloramphenicol was measured in strain 249-2 by introduction of a plasmid which encodes production of chloramphenicol acetyltransferase. After 1 h of incubation, 18.5% +/- 1.54% versus 70.1% +/- 3.52%, and after 2 h, 4.20% +/- 1.65% versus 41.90% +/- 2.16% remained in supernatants from organisms grown in the absence and presence of 10 mM salicylate, respectively. Outer membrane protein pattern analysis demonstrated the absence of a protein of apparent molecular weight of 40,000 when strain 249-2 was grown in the presence of 10 mM salicylate. To determine whether this protein functioned as a porin, reconstituted membrane vesicles were constructed to assess antibiotic permeability. Vesicles constructed with this salicylate-suppressible outer membrane protein (OpcS) were permeable to chloramphenicol but not to penicillin G. These findings suggest that OpcS is a selective, antibiotic-permeable porin which can be suppressed by growth in the presence of salicylate. Further investigation will be required to determine the biochemical effects of salicylate on porin synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Matsuyama S., Mizuno T., Mizushima S. Function of micF as an antisense RNA in osmoregulatory expression of the ompF gene in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3007–3012. doi: 10.1128/jb.169.7.3007-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff S. C., Labrozzi P. H. Differences in drug susceptibility between isolates of Pseudomonas cepacia recovered from patients with cystic fibrosis and other sources and its relationship to beta-lactamase focusing pattern. Pediatr Pulmonol. 1986 Nov-Dec;2(6):368–372. doi: 10.1002/ppul.1950020609. [DOI] [PubMed] [Google Scholar]
- Aronoff S. C. Outer membrane permeability in Pseudomonas cepacia: diminished porin content in a beta-lactam-resistant mutant and in resistant cystic fibrosis isolates. Antimicrob Agents Chemother. 1988 Nov;32(11):1636–1639. doi: 10.1128/aac.32.11.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumercier M., Murray D. M., Rosner J. L. Potentiation of susceptibility to aminoglycosides by salicylate in Escherichia coli. Antimicrob Agents Chemother. 1990 May;34(5):786–791. doi: 10.1128/aac.34.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman W., Lessie T. G. Response of Pseudomonas cepacia to beta-Lactam antibiotics: utilization of penicillin G as the carbon source. J Bacteriol. 1979 Dec;140(3):1126–1128. doi: 10.1128/jb.140.3.1126-1128.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Hedin L. A., Lien D. M. Chloramphenicol resistance in Pseudomonas cepacia because of decreased permeability. Antimicrob Agents Chemother. 1989 Feb;33(2):136–141. doi: 10.1128/aac.33.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Lien D. M., Hedin L. A. Isolation and characterization of dihydrofolate reductase from trimethoprim-susceptible and trimethoprim-resistant Pseudomonas cepacia. Antimicrob Agents Chemother. 1989 Aug;33(8):1247–1251. doi: 10.1128/aac.33.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C., Labrozzi P. H., Aronoff S. C. Decreased baseline beta-lactamase production and inducibility associated with increased piperacillin susceptibility of Pseudomonas cepacia isolated from children with cystic fibrosis. Pediatr Res. 1986 Nov;20(11):1174–1177. doi: 10.1203/00006450-198611000-00026. [DOI] [PubMed] [Google Scholar]
- Chopra I., Eccles S. J. Diffusion of tetracycline across the outer membrane of Escherichia coli K-12: involvement of protein Ia. Biochem Biophys Res Commun. 1978 Jul 28;83(2):550–557. doi: 10.1016/0006-291x(78)91025-2. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederer G. M., Matsen J. M. Colonization and infection with Pseudomonas cepacia. J Infect Dis. 1972 Jun;125(6):613–618. doi: 10.1093/infdis/125.6.613. [DOI] [PubMed] [Google Scholar]
- Foulds J., Murray D. M., Chai T., Rosner J. L. Decreased permeation of cephalosporins through the outer membrane of Escherichia coli grown in salicylates. Antimicrob Agents Chemother. 1989 Apr;33(4):412–417. doi: 10.1128/aac.33.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Jin E., Levison H., Isles A., Fleming P. C. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):331–336. doi: 10.1093/jac/12.suppl_a.331. [DOI] [PubMed] [Google Scholar]
- Gold R., Overmeyer A., Knie B., Fleming P. C., Levison H. Controlled trial of ceftazidime vs. ticarcillin and tobramycin in the treatment of acute respiratory exacerbations in patients with cystic fibrosis. Pediatr Infect Dis. 1985 Mar-Apr;4(2):172–177. doi: 10.1097/00006454-198503000-00012. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Role of porins in outer membrane permeability. J Bacteriol. 1987 Mar;169(3):929–933. doi: 10.1128/jb.169.3.929-933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wong P. G. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984 Jul;26(1):48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. C., Ederer G. M., Matsen J. M. Contamination of commercially packaged urinary catheter kits with the pseudomonad EO-1. N Engl J Med. 1970 Jan 1;282(1):33–35. doi: 10.1056/NEJM197001012820108. [DOI] [PubMed] [Google Scholar]
- Hirai K., Iyobe S., Inoue M., Mitsuhashi S. Purification and properties of a new beta-lactamase from Pseudomonas cepacia. Antimicrob Agents Chemother. 1980 Mar;17(3):355–358. doi: 10.1128/aac.17.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Kanamaru K., Aiba H., Mizuno T. Transmembrane signal transduction and osmoregulation in Escherichia coli: I. Analysis by site-directed mutagenesis of the amino acid residues involved in phosphotransfer between the two regulatory components, EnvZ and OmpR. J Biochem. 1990 Sep;108(3):483–487. doi: 10.1093/oxfordjournals.jbchem.a123225. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martone W. J., Osterman C. A., Fisher K. A., Wenzel R. P. Pseudomonas cepacia: implications and control of epidemic nosocomial colonization. Rev Infect Dis. 1981 Jul-Aug;3(4):708–715. doi: 10.1093/clinids/3.4.708. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Inokuchi K., Mizushima S. Promoter exchange between ompF and ompC, genes for osmoregulated major outer membrane proteins of Escherichia coli K-12. J Bacteriol. 1984 Jun;158(3):1041–1047. doi: 10.1128/jb.158.3.1041-1047.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Mizushima S. Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes. Mol Microbiol. 1990 Jul;4(7):1077–1082. doi: 10.1111/j.1365-2958.1990.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Hancock R. E. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob Agents Chemother. 1986 Dec;30(6):923–926. doi: 10.1128/aac.30.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara F., Inokuchi K., Matsuyama S., Mizushima S. Mutation causing reverse osmoregulation of synthesis of OmpF, a major outer membrane protein of Escherichia coli. J Bacteriol. 1984 Aug;159(2):688–692. doi: 10.1128/jb.159.2.688-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y., Mizushima S. Regulation of outer membrane porin protein synthesis in Escherichia coli K-12: ompF regulates the expression of ompC. J Bacteriol. 1983 May;154(2):669–675. doi: 10.1128/jb.154.2.669-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Moore R. A., Moore L. V., Hancock R. E. Role of porins in intrinsic antibiotic resistance of Pseudomonas cepacia. Antimicrob Agents Chemother. 1987 Jan;31(1):121–123. doi: 10.1128/aac.31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A., Wood M. S., Cacalano G. S., Chin N. X. Isolation and characterization of a penicillinase from Pseudomonas cepacia 249. Antimicrob Agents Chemother. 1988 Jun;32(6):838–843. doi: 10.1128/aac.32.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein B. J., Hall D. E. Pneumonia and septicemia due to Pseudomonas cepacia in a patient with cystic fibrosis. Johns Hopkins Med J. 1980 Nov;147(5):188–189. [PubMed] [Google Scholar]
- Rosner J. L., Chai T. J., Foulds J. Regulation of ompF porin expression by salicylate in Escherichia coli. J Bacteriol. 1991 Sep;173(18):5631–5638. doi: 10.1128/jb.173.18.5631-5638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Nonheritable resistance to chloramphenicol and other antibiotics induced by salicylates and other chemotactic repellents in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8771–8774. doi: 10.1073/pnas.82.24.8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Garrett S., Sodergren E., Silhavy T. J. Mutations that define the promoter of ompF, a gene specifying a major outer membrane porin protein. J Bacteriol. 1985 Jun;162(3):1054–1060. doi: 10.1128/jb.162.3.1054-1060.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokishita S., Yamada H., Aiba H., Mizuno T. Transmembrane signal transduction and osmoregulation in Escherichia coli: II. The osmotic sensor, EnvZ, located in the isolated cytoplasmic membrane displays its phosphorylation and dephosphorylation abilities as to the activator protein, OmpR. J Biochem. 1990 Sep;108(3):488–493. doi: 10.1093/oxfordjournals.jbchem.a123226. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Muszynski M. J., Pai C. H., Marcon M. J., Hribar M. M., Gilligan P. H., Matsen J. M., Ahlin P. A., Hilman B. C., Chartrand S. A. Selective and differential medium for recovery of Pseudomonas cepacia from the respiratory tracts of patients with cystic fibrosis. J Clin Microbiol. 1987 Sep;25(9):1730–1734. doi: 10.1128/jcm.25.9.1730-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]