Abstract
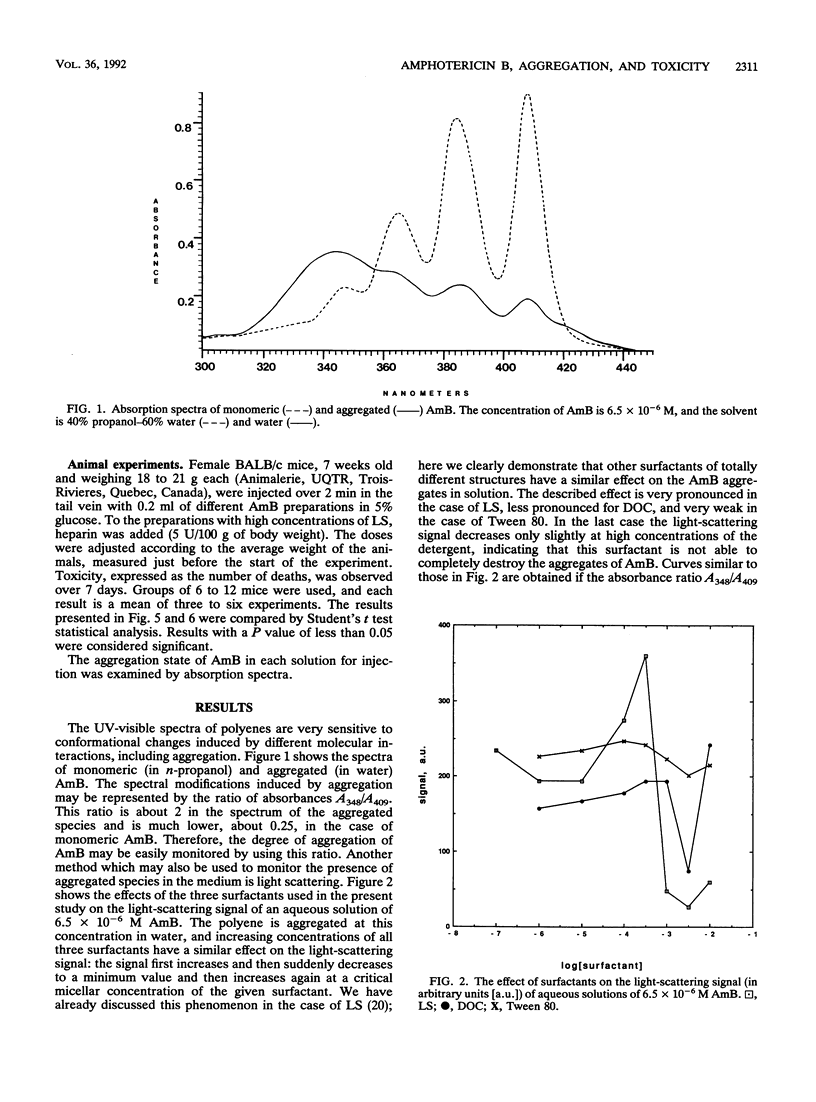
Amphotericin B (AmB) is a very effective antifungal agent for most systemic fungal infections. However, the relatively high toxicity of this drug imposes limits on its clinical usefulness. Most of the current work in this field is devoted to the search for less-toxic formulations of the drug. Here we describe the effects of three surfactants, one anionic and the other two nonionic, on the aggregation state of AmB in solutions which were injected intravenously into mice. The degree of aggregation of AmB was monitored spectroscopically and by light scattering. The toxicity was expressed as percentage of survivors. These results were compared with those obtained with doses of AmB the same as those present in a commercial formulation of AmB, Fungizone. Two surfactants, lauryl sucrose and sodium deoxycholate, used at concentrations which induced monomerization of AmB, substantially decreased the acute toxicity of AmB to mice. Conversely, the third surfactant, Tween 80, showed a synergistic potentiation of the toxicity of the antibiotic. A good correlation was found between the in vivo toxicity and the aggregation state of AmB in injected solutions. Solutions in which AmB was almost entirely monomeric were half as toxic after 24 h and about six times less toxic after 1 week than the corresponding solutions of Fungizone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler-Moore J. P., Chiang S. M., Satorius A., Guerra D., McAndrews B., McManus E. J., Proffitt R. T. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J Antimicrob Chemother. 1991 Oct;28 (Suppl B):63–71. doi: 10.1093/jac/28.suppl_b.63. [DOI] [PubMed] [Google Scholar]
- Barwicz J., Gareau R., Audet A., Morisset A., Villiard J., Gruda I. Inhibition of the interaction between lipoproteins and amphotericin B by some delivery systems. Biochem Biophys Res Commun. 1991 Dec 16;181(2):722–728. doi: 10.1016/0006-291x(91)91250-g. [DOI] [PubMed] [Google Scholar]
- Bolard J., Legrand P., Heitz F., Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991 Jun 11;30(23):5707–5715. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- Chopra R., Blair S., Strang J., Cervi P., Patterson K. G., Goldstone A. H. Liposomal amphotericin B (AmBisome) in the treatment of fungal infections in neutropenic patients. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):93–104. doi: 10.1093/jac/28.suppl_b.93. [DOI] [PubMed] [Google Scholar]
- Davis S. S., Washington C., West P., Illum L., Liversidge G., Sternson L., Kirsh R. Lipid emulsions as drug delivery systems. Ann N Y Acad Sci. 1987;507:75–88. doi: 10.1111/j.1749-6632.1987.tb45793.x. [DOI] [PubMed] [Google Scholar]
- Diamond R. D. The growing problem of mycoses in patients infected with the human immunodeficiency virus. Rev Infect Dis. 1991 May-Jun;13(3):480–486. doi: 10.1093/clinids/13.3.480. [DOI] [PubMed] [Google Scholar]
- Gruda I., Dussault N. Effect of the aggregation state of amphotericin B on its interaction with ergosterol. Biochem Cell Biol. 1988 Mar;66(3):177–183. doi: 10.1139/o88-024. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Grant C. W., Barber K. R., Kalp M. A. Mechanism of the selective toxicity of amphotericin B incorporated into liposomes. Mol Pharmacol. 1987 Jan;31(1):1–11. [PubMed] [Google Scholar]
- Lopez-Berestein G., Bodey G. P., Frankel L. S., Mehta K. Treatment of hepatosplenic candidiasis with liposomal-amphotericin B. J Clin Oncol. 1987 Feb;5(2):310–317. doi: 10.1200/JCO.1987.5.2.310. [DOI] [PubMed] [Google Scholar]
- Meunier F., Prentice H. G., Ringdén O. Liposomal amphotericin B (AmBisome): safety data from a phase II/III clinical trial. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):83–91. doi: 10.1093/jac/28.suppl_b.83. [DOI] [PubMed] [Google Scholar]
- Proffitt R. T., Satorius A., Chiang S. M., Sullivan L., Adler-Moore J. P. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J Antimicrob Chemother. 1991 Oct;28 (Suppl B):49–61. doi: 10.1093/jac/28.suppl_b.49. [DOI] [PubMed] [Google Scholar]
- Sabra R., Branch R. A. Amphotericin B nephrotoxicity. Drug Saf. 1990 Mar-Apr;5(2):94–108. doi: 10.2165/00002018-199005020-00003. [DOI] [PubMed] [Google Scholar]
- Szoka F. C., Jr, Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1987 Mar;31(3):421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancrède P., Barwicz J., Jutras S., Gruda I. The effect of surfactants on the aggregation state of amphotericin B. Biochim Biophys Acta. 1990 Dec 14;1030(2):289–295. doi: 10.1016/0005-2736(90)90305-8. [DOI] [PubMed] [Google Scholar]
- Tasset C., Préat V., Roland M. The influence of Myrj 59 on the solubility, toxicity and activity of amphotericin B. J Pharm Pharmacol. 1991 May;43(5):297–302. doi: 10.1111/j.2042-7158.1991.tb06693.x. [DOI] [PubMed] [Google Scholar]



