Abstract
Transposition of TnV and Tn5lac into Myxococcus xanthus yielded 8,381 kanamycin-resistant mutants that were tested for antibiotic TA production. Twenty-four of the mutants were nonproducers of TA (less than 0.4 ng/ml), and 3 produced a higher level (2.5 micrograms/ml) than the parent strain (1.5 micrograms/ml). For most of the strains, there was 100% cotransduction between kanamycin resistance and the altered TA phenotype. Southern blot analysis of restriction digests of the mutant DNA indicated that the transposons were inserted at different sites on the M. xanthus chromosome. The TA genes were mapped by cotransduction between pairs of mutants following replacement of the initial insert of one of the pair with the tetracycline resistance transposon Tn5-132. Nine of the 13 nonproducers tested were linked over a 36-kb stretch of the chromosome. There was no linkage between one of the overproducers and any of the nonproducers tested.
Full text
PDF
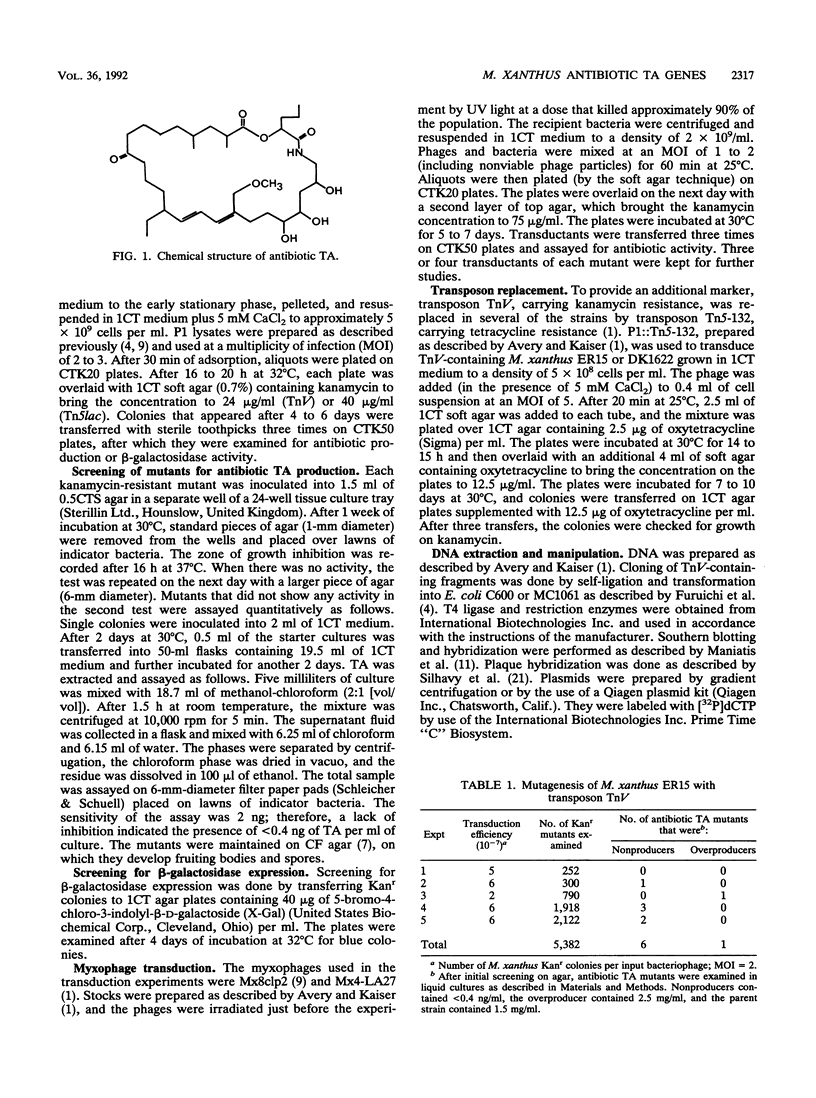
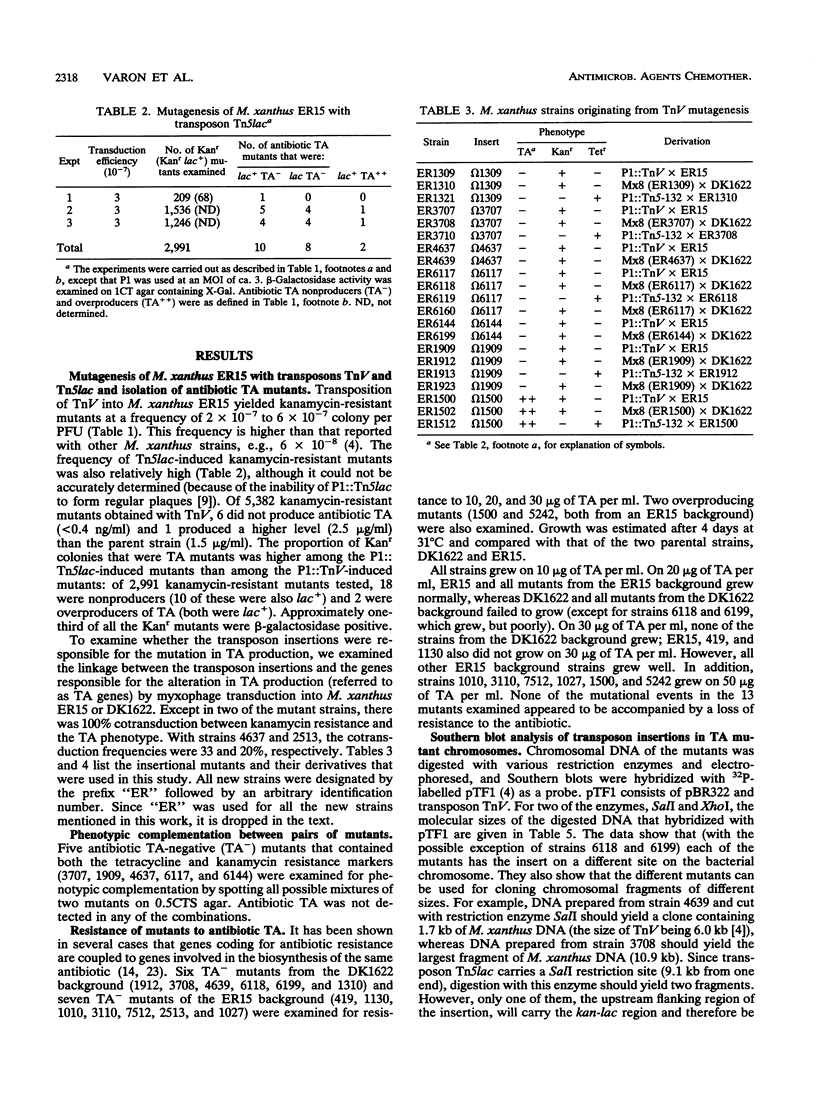
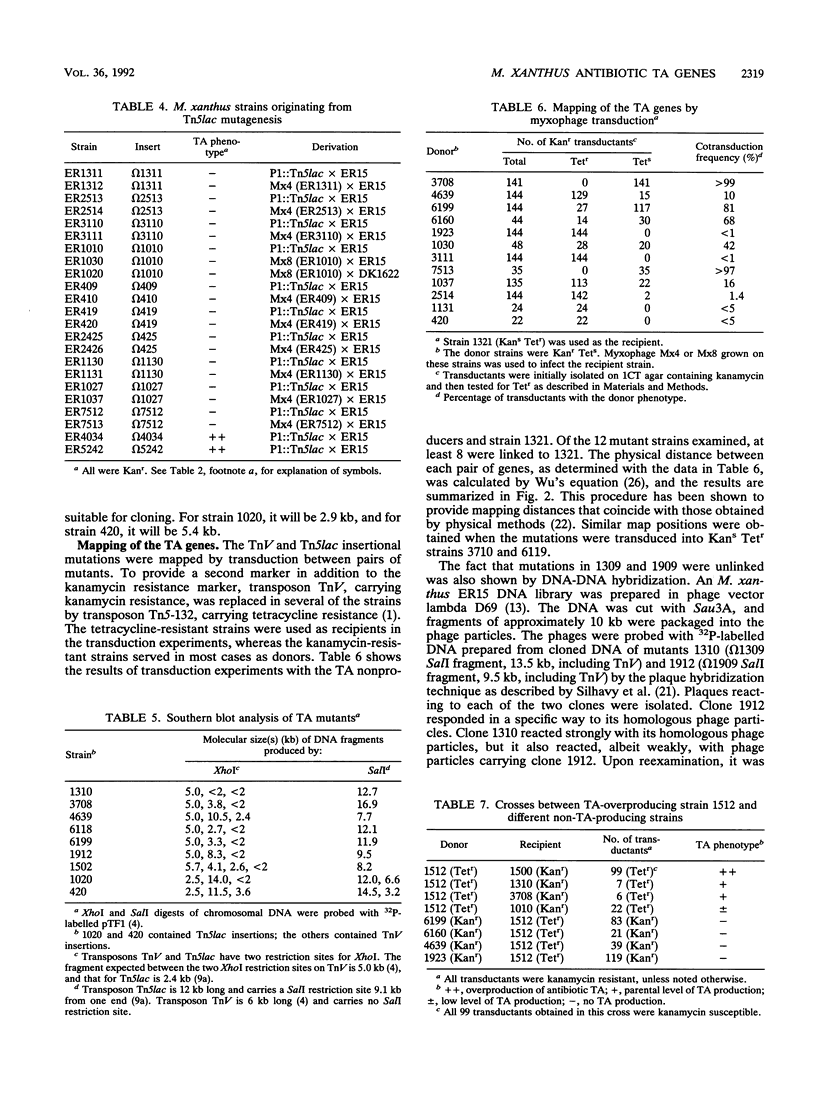


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery L., Kaiser D. In situ transposon replacement and isolation of a spontaneous tandem genetic duplication. Mol Gen Genet. 1983;191(1):99–109. doi: 10.1007/BF00330896. [DOI] [PubMed] [Google Scholar]
- Chen H. W., Kuspa A., Keseler I. M., Shimkets L. J. Physical map of the Myxococcus xanthus chromosome. J Bacteriol. 1991 Mar;173(6):2109–2115. doi: 10.1128/jb.173.6.2109-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Keseler I. M., Shimkets L. J. Genome size of Myxococcus xanthus determined by pulsed-field gel electrophoresis. J Bacteriol. 1990 Aug;172(8):4206–4213. doi: 10.1128/jb.172.8.4206-4213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T., Inouye M., Inouye S. Novel one-step cloning vector with a transposable element: application to the Myxococcus xanthus genome. J Bacteriol. 1985 Oct;164(1):270–275. doi: 10.1128/jb.164.1.270-275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fytlovitch S., Nathan P. D., Zafriri D., Rosenberg E. Amino acid precursors of Myxococcus xanthus antibiotic TA. J Antibiot (Tokyo) 1983 Nov;36(11):1525–1530. doi: 10.7164/antibiotics.36.1525. [DOI] [PubMed] [Google Scholar]
- Gerth K., Irschik H., Reichenbach H., Trowitzsch W. The myxovirescins, a family of antibiotics from Myxococcus virescens (Myxobacterales). J Antibiot (Tokyo) 1982 Nov;35(11):1454–1459. doi: 10.7164/antibiotics.35.1454. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Introduction of transposon Tn5 into Myxococcus for analysis of developmental and other nonselectable mutants. Proc Natl Acad Sci U S A. 1981 Jan;78(1):425–429. doi: 10.1073/pnas.78.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor A., Eli I., Varon M., Judes H., Rosenberg E. Effect of adhesive antibiotic TA on plaque and gingivitis in man. J Clin Periodontol. 1989 Nov;16(10):621–624. doi: 10.1111/j.1600-051x.1989.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Ward D. F. A bacteriophage lambda vector for cloning with BamHI and Sau3A. Gene. 1982 Dec;20(3):317–322. doi: 10.1016/0378-1119(82)90200-1. [DOI] [PubMed] [Google Scholar]
- Motamedi H., Hutchinson C. R. Cloning and heterologous expression of a gene cluster for the biosynthesis of tetracenomycin C, the anthracycline antitumor antibiotic of Streptomyces glaucescens. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4445–4449. doi: 10.1073/pnas.84.13.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor K. A., Zusman D. R. Genetic analysis of tag mutants of Myxococcus xanthus provides evidence for two developmental aggregation systems. J Bacteriol. 1990 Jul;172(7):3868–3878. doi: 10.1128/jb.172.7.3868-3878.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Fytlovitch S., Carmeli S., Kashman Y. Chemical properties of Myxococcus xanthus antibiotic TA. J Antibiot (Tokyo) 1982 Jul;35(7):788–793. doi: 10.7164/antibiotics.35.788. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Vaks B., Zuckerberg A. Bactericidal action of an antibiotic produced by Myxococcus xanthus. Antimicrob Agents Chemother. 1973 Nov;4(5):507–513. doi: 10.1128/aac.4.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Gill R. E., Kaiser D. Developmental cell interactions in Myxococcus xanthus and the spoC locus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1406–1410. doi: 10.1073/pnas.80.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergren E., Cheng Y., Avery L., Kaiser D. Recombination in the Vicinity of Insertions of Transposon Tn 5 in MYXOCOCCUS XANTHUS. Genetics. 1983 Oct;105(2):281–291. doi: 10.1093/genetics/105.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaks B., Zuckerberg A., Rosenberg E. Purification and partial characterization of an antibiotic produced by Myxococcus xanthus. Can J Microbiol. 1974 Feb;20(2):155–161. doi: 10.1139/m74-025. [DOI] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafriri D., Rosenberg E., Mirelman D. Mode of action of Myxococcus xanthus antibiotic TA. Antimicrob Agents Chemother. 1981 Feb;19(2):349–351. doi: 10.1128/aac.19.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]



