Abstract
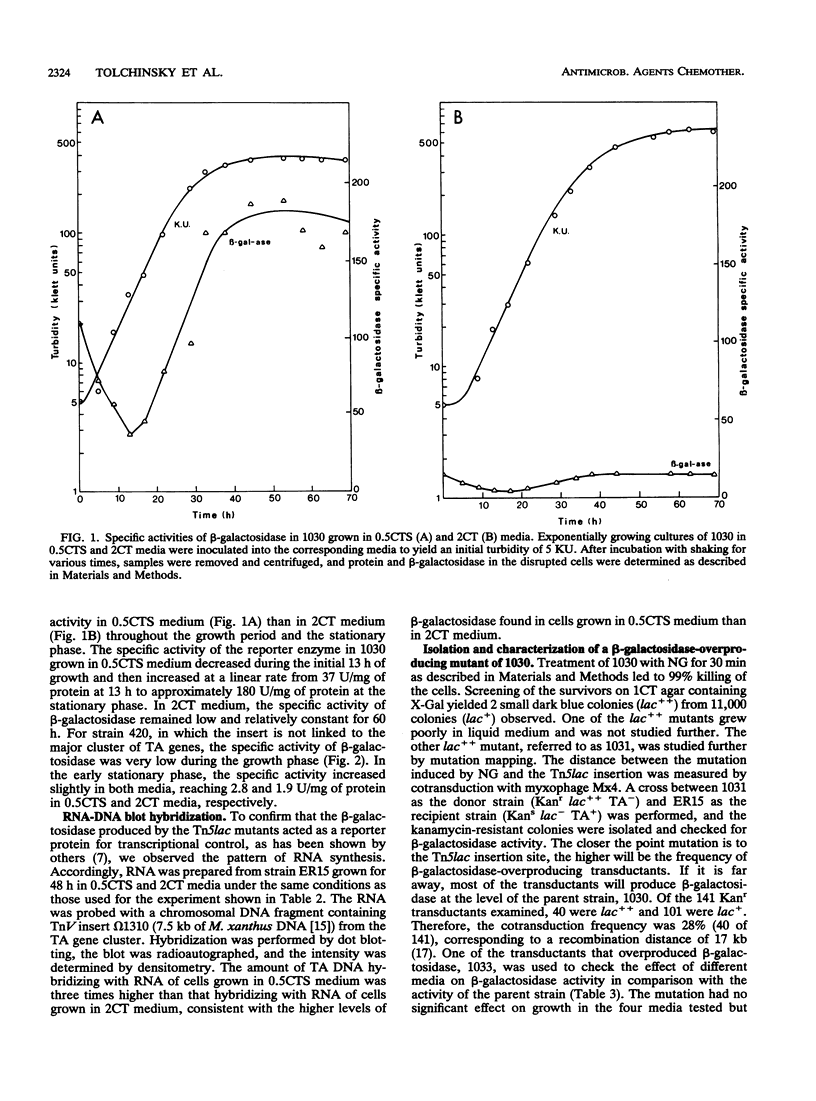
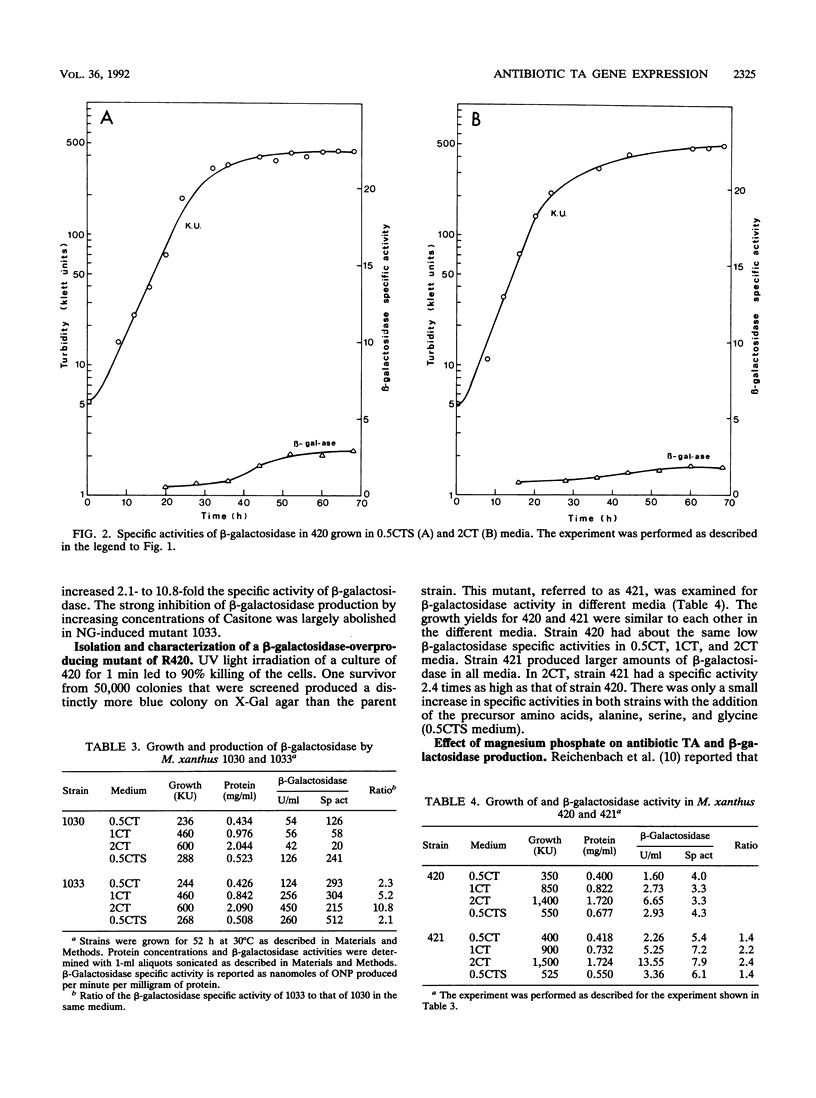
The beta-galactosidase activities arising from Tn5lac insertions in several genes required for antibiotic TA production were measured under different growth conditions. In all of the non-TA-producing mutants, the beta-galactosidase specific activity was higher when the cells were grown in nutrient-limited 0.5CTS medium (0.5% Casitone plus alanine, serine, and glucose) than in rich 2CT medium (2% Casitone). One of the mutants, 420, had low beta-galactosidase specific activity in both media. The other seven mutants containing inserts in genes essential for TA production had specific activities of 139 to 367 U/mg of protein in 0.5CTS medium and 11 to 48 U/mg of protein in 2CT medium. The beta-galactosidase specific activities of two strains, 1030 and 420, increased during exponential growth in 0.5CTS medium. The beta-galactosidase specific activities of both strains increased greatly when the cells were grown in the presence of magnesium phosphate, which traps ammonium ions. The Tn5lac insertions in 1030 and 420 were used to screen for mutants with increased levels of transcription. An N-methyl-N'-nitro-N-nitrosoguanidine-induced mutation in 1030 that mapped 17 kb from the omega 1010 insert increased the specific activity of beta-galactosidase 21 times in 2CT medium. The regulatory mutation appears to release the repression caused by 2CT medium. A UV-induced mutation in 420 increased the beta-galactosidase specific activity 1.4 to 2.4 times. Medium conditions that affect the transcription of TA genes are discussed in terms of enhanced antibiotic TA production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery L., Kaiser D. In situ transposon replacement and isolation of a spontaneous tandem genetic duplication. Mol Gen Genet. 1983;191(1):99–109. doi: 10.1007/BF00330896. [DOI] [PubMed] [Google Scholar]
- Binnie C., Warren M., Butler M. J. Cloning and heterologous expression in Streptomyces lividans of Streptomyces rimosus genes involved in oxytetracycline biosynthesis. J Bacteriol. 1989 Feb;171(2):887–895. doi: 10.1128/jb.171.2.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen H. W., Kuspa A., Keseler I. M., Shimkets L. J. Physical map of the Myxococcus xanthus chromosome. J Bacteriol. 1991 Mar;173(6):2109–2115. doi: 10.1128/jb.173.6.2109-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fytlovitch S., Nathan P. D., Zafriri D., Rosenberg E. Amino acid precursors of Myxococcus xanthus antibiotic TA. J Antibiot (Tokyo) 1983 Nov;36(11):1525–1530. doi: 10.7164/antibiotics.36.1525. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E., Vaks B., Zuckerberg A. Bactericidal action of an antibiotic produced by Myxococcus xanthus. Antimicrob Agents Chemother. 1973 Nov;4(5):507–513. doi: 10.1128/aac.4.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaks B., Zuckerberg A., Rosenberg E. Purification and partial characterization of an antibiotic produced by Myxococcus xanthus. Can J Microbiol. 1974 Feb;20(2):155–161. doi: 10.1139/m74-025. [DOI] [PubMed] [Google Scholar]
- Varon M., Fuchs N., Monosov M., Tolchinsky S., Rosenberg E. Mutation and mapping of genes involved in production of the antibiotic TA in Myxococcus xanthus. Antimicrob Agents Chemother. 1992 Oct;36(10):2316–2321. doi: 10.1128/aac.36.10.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Zusman D. R. Evidence that the Myxococcus xanthus frz genes are developmentally regulated. J Bacteriol. 1989 Nov;171(11):6174–6186. doi: 10.1128/jb.171.11.6174-6186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]


