Abstract
Polyaromatic hydrocarbons are ubiquitous environmental chemicals that are important mutagens and carcinogens. The purpose of this study was to determine whether genes within the major histocompatibility complex (MHC) influence their biological activities. Cell-mediated immunity to dimethylbenz(a)anthracene (DMBA) was investigated in congenic strains of mice. On three different backgrounds, H-2k and H-2a haplotype mice developed significantly greater contact-hypersensitivity responses to DMBA than H-2b, H-2d, and H-2s mice. In B10.A(R1) mice, which are Kk and Id, a vigorous contact-hypersensitivity response was present, indicating that the response was governed by class I, rather than class II, MHC genes. C3H/HeN (H-2k) and C3H.SW (H-2s) strains were also compared for the development of skin tumors and the persistence of DMBA–DNA adducts. When subjected to a DMBA initiation, phorbol 12-tetradecanoate 13-acetate (TPA)-promotion skin-tumorigenesis protocol, C3H/HeN mice, (which develop cell-mediated immunity to DMBA) were found to have significantly fewer tumors than C3H.SW mice (a strain that failed to develop a cell-mediated immune response to DMBA). DMBA–DNA adducts were removed more rapidly in C3H/HeN than in C3H.SW mice. The results indicate that genes within the MHC play an important role in several of the biological activities of carcinogenic polyaromatic hydrocarbons. The observations are consistent with the hypothesis that cell-mediated immunity to chemical carcinogens serves to protect individuals by removing mutant cells before they can evolve into clinically apparent neoplasms.
Polyaromatic hydrocarbons (PAHs) are common environmental pollutants found in automobile emissions, tobacco smoke, charcoal-broiled food, and chimney soot. Many of these agents are important mutagens and carcinogens in human and in experimental animal models (1). In animals, they have been employed as prototypic agents to better define the mechanisms by which chemicals cause cancer. The PAHs dimethylbenz(a)anthracene (DMBA), benzo[a]pyrene, and 3-methylcholanthrene are all carcinogenic in murine systems. The skin-tumor system has been particularly helpful in investigating these issues (1).
PAHs are relatively inert compounds that are metabolized via cytochrome P450-dependent monoxygenases (2). Reactive intermediates, rather than the parent compound, are the agents that bind to DNA (1–3), and it is a diol, epoxide metabolite that is the actual mutagenic and carcinogenic moiety. H-ras oncogene mutations are particularly important for the carcinogenic activity of PAHs (4).
Although it is clear that PAHs are carcinogenic, it is important to note that not all strains of mice exposed to these agents develop tumors (3). At least part of the variation in susceptibility to PAH-induced cancer relates to genetic differences in the Ah receptor locus (1, 3). Mice that carry the b, or high-affinity, allele efficiently convert PAHs to the carcinogenic diol, epoxide metabolite, whereas those that carry the d allele do not (3, 5). Strains of mice with the d allele develop far fewer tumors when subjected to PAH-induced carcinogenesis protocols than do animals with the b allele. Several other genetic loci are involved in PAH-induced tumorigenesis both at the initiation stage and during tumor promotion (6). Few of these loci have been carefully defined, however.
PAHs also have significant interactions with the immune system (7–13). DMBA, benzo[a]pyrene, and 3-methylcholanthrene are all contact allergens when applied to the skin (7, 8, 11–13). Like several other contact allergens, induction of contact hypersensitivity to DMBA requires Langerhans cells and is mediated by CD8+ T cells (8). Furthermore, the same enzymatic pathways that are required for the mutagenic and carcinogenic activities of PAHs must also be fully operational for contact sensitization to occur (8). For example, pharmacologic inhibition of cytochrome P450-dependent enzyme metabolism blocks the DMBA contact-hypersensitivity response, and all strains of mice that express the d allele at the Ah receptor locus (the allele that is associated with a deficiency in the ability to metabolize PAHs) fail to develop contact hypersensitivity to DMBA.
Although expression of the appropriate allele at the Ah locus is one determinant of susceptibility to PAH contact hypersensitivity, we wanted to determine whether polymorphisms at other genetic loci were involved as well. In this study, we have found that the induction of DMBA contact hypersensitivity cosegregates with genes within the major histocompatibility complex (MHC). Moreover, our studies indicate that the same MHC genes that identify genetic loci are also associated with resistance to DMBA-induced skin cancer and more efficient removal of DMBA–DNA adducts.
MATERIALS AND METHODS
Mice.
C3H/HeN mice were purchased from Charles River Breeding Laboratories. C3H/HeJ, C57BL/6, C57BL/10, B10.D2, A/J, A/SW, A.BY, C3H.SW, and C3HxDBA/2 mice were obtained from The Jackson Laboratory. B10.BR and B10.S mice were the kind gift of Chella David (Department of Immunology, Mayo Clinic, Rochester, MN).
Chemicals.
DMBA was purchased from Aldrich. TPA and d,l-ornithine were obtained from Sigma. d,l-[14C]Ornithine (specific activity 58 Ci/mmol; 1 Ci = 37 GBq) was purchased from New England Nuclear. All chemicals were of the highest purity commercially available.
DMBA Contact Sensitization.
Immunization and elicitation of DMBA contact hypersensitivity were carried out according to described methodologies (7, 8). Briefly, animals were sensitized to DMBA by applying 100 μl of a 0.1% solution to the shaved abdominal skin and covering the painted area with a water- and vapor-permeable membrane (BioOcclusive Bandage, Johnson & Johnson Medical). Five days later, the response was elicited by painting the ears of mice with 20 μl of a 0.1% solution of DMBA. Contact hypersensitivity was assessed by measuring ears before elicitation and daily for up to 5 days after elicitation with a dial thickness gauge micrometer (Mitutoya, Tokyo, Japan). The maximum increment in ear thickness compared with the baseline preelicitation level was used to quantify the magnitude of the response. Negative controls were ear-challenged with DMBA but were not sensitized on the abdomen. There were three to five mice in all of the panels tested.
Skin Tumorigenesis.
A two-stage skin tumorigenesis protocol in which DMBA was the initiating agent and TPA was the promoter was employed, using methods that have been described (14). Briefly, the dorsal skin of mice (20 mice per panel) was painted with 100 μg of DMBA (0.1% wt/vol in acetone). Beginning 1 week later, TPA (40 nmol) was applied biweekly to the site that had been treated previously with DMBA. Mice were evaluated weekly for tumors. Only tumors that had attained a size of 1 mm or greater and that were present for 2 weeks or longer were counted.
TPA-Induced Ear Swelling.
Baseline mouse-ear thickness measurements were made with an engineer’s micrometer. The ears were then painted with 32.4 nmol of TPA. The ears were again measured 6 and 24 hr later, and the increment in ear thickness compared with baseline levels was used to quantify the inflammatory response caused by TPA.
TPA Induction of Ornithine Decarboxylase Activity.
TPA (40 nmol) was applied to the skin, and epidermal 100,000 × g supernatant fractions were prepared 12 and 24 hr later. Ornithine decarboxylase activity was assessed by measuring 14CO2 production from d,l-[14C]ornithine.
Quantitation of DMBA–DNA Adducts.
[3H]DMBA (100 μg) was applied to the skin. At the indicated time (see Results) epidermal homogenates were prepared, and DNA was isolated and purified. RNA was removed by treatment with RNase A (1,000 units/ml). The amount of [3H]DMBA bound to DNA was evaluated by scintillation spectrometry.
RESULTS
The MHC Influences Development of Cell-Mediated Immune Responses to the Carcinogenic PAH DMBA.
In previous studies, we have shown that metabolism via the cytochrome P450-dependent enzyme system is an absolute precondition for the development of cell-mediated immunity to carcinogenic PAHs (8). Animals that express the d allele of the Ah receptor have a relative inability to metabolize PAHs (2, 5). Allergic contact hypersensitivity does not occur in Ahd strains of mice (DBA/2, AKR/J, SJL/J, and RF/J) (8). To determine whether the Ah locus was the only genetic locus necessary for the induction of DMBA contact hypersensitivity, several strains of Ahb strains of mice, all of which were found in preliminary experiments (C.A.E. and H.M., unpublished data) to metabolize PAHs normally, were examined for their ability to develop contact hypersensitivity to DMBA. Although C3H/HeN, C3H/HeJ, A/J, and C3HxDBA/2 mice could be contact-sensitized to this PAH, C57BL/6 and C57BL/10 mice could not (Table 1). This was a consistent and reproducible finding. On the other hand, both strains could be immunized to the unrelated hapten dinitrofluorobenzene (data not shown, and refs. 15 and 16), indicating that the lack of a cell-mediated immune response to DMBA was not caused by an inherent inability to mount contact-hypersensitivity reactions. From these experiments it was possible to conclude that, although the Ahb receptor was necessary for induction of DMBA contact hypersensitivity, it alone was not sufficient for its development and that a second genetic locus must also be involved.
Table 1.
DMBA contact sensitization of Ahb mice
| Strain | MHC haplotype
|
Positive control | Negative control | Net ear-swelling response | |||
|---|---|---|---|---|---|---|---|
| K | I | S | D | ||||
| C3H/HeN | k | k | k | k | 15.2 ± 3.0 | 0.6 ± 0.2 | 14.6 |
| A/J | k | k | d | d | 13.2 ± 2.8 | 0.0 ± 0.1 | 13.2 |
| C3HxDBA/2 | k/d | k/d | k/d | k/d | 7.1 ± 0.7 | 0.4 ± 0.5 | 6.7 |
| Sencar | outbred | 12.9 ± 4.6 | −0.5 ± 0.7 | 13.4 | |||
| C57BL/6 | b | b | b | b | 1.6 ± 0.6 | 3.2 ± 0.5 | −1.6 |
| C57BL/10 | b | b | b | b | 0.2 ± 0.1 | 0.1 ± 0.2 | 0.1 |
| B10.S | s | s | s | s | 2.6 ± 1.0 | 1.4 ± 0.4 | 1.2 |
| B10.D2 | d | d | d | d | 1.5 ± 0.4 | 0.5 ± 0.1 | 1.0 |
| B10.Q | q | q | q | q | 2.6 ± 0.3 | 0.3 ± 0.1 | 2.3 |
| B10.BR | k | k | k | k | 5.7 ± 1.7 | 0.4 ± 0.5 | 5.3 |
| B10.A | k | k | d | d | 8.1 ± 2.8 | −0.2 ± 0.5 | 8.3 |
| B10.A(R1) | k | d | d | d | 20.1 ± 4.0 | −1.2 ± 0.9 | 21.3 |
| B10.TL | s | k | k | d | 0.6 ± 0.6 | 0.9 ± 0.4 | −0.3 |
| B10.AQR | q | k | d | d | 0.9 ± 0.3 | 0.2 ± 0.3 | 0.7 |
Positive controls were sensitized and ear challenged with DMBA; negative controls were ear challenged only.
Because allergic contact hypersensitivity is an immunological phenomenon and because many of the activities of the immune system are governed by the MHC, experiments were next conducted to investigate whether the MHC could exert genetic control over development of DMBA contact hypersensitivity. To do this, congenic strains of mice on C3H- and A-strain backgrounds were sensitized and ear-challenged to DMBA. When C3H/HeN (H-2k) and C3H.SW (H-2b) mice were compared, C3H/HeN mice developed a substantial contact hypersensitivity response, but C3H.SW mice did not (Fig. 1). A similar situation was observed with respect to DMBA contact hypersensitivity in A-strain mice. A/J (H-2a) mice sensitized to DMBA developed significantly greater ear swelling than did A.BY (H-2b) or A.SW (H-2s) mice (Fig. 1). Although the overall magnitude of the DMBA contact-hypersensitivity reaction was smaller on the C57BL/10 background than on either the C3H- or A-strain backgrounds, noticeable differences in ear swelling were observed on this background as well (Table 1). B10.BR (H-2k) mice developed significantly more ear swelling than B10.S (H-2s), B10.D2 (H-2d), or C57BL/10 (H-2b) mice. In all three backgrounds examined (C3H, A strain, and C57BL/10), H-2k congenic mice developed a greater DMBA contact hypersensitivity response than H-2d mice; thus, the results of these studies indicated that polymorphisms within the MHC were, in fact, responsible for variations in the development of DMBA contact hypersensitivity.
Figure 1.
DMBA contact-hypersensitivity response in C3H and A-strain mice. Panels of mice that were sensitized to 100 μl of DMBA and were ear-challenged with 20 μl of DMBA served as positive controls. Panels of mice that were only ear-challenged with 20 μl of DMBA served as negative controls. Positive controls are the solid bars; negative controls are the shaded bars.
DMBA contact hypersensitivity was also examined in several other strains of mice to further delineate the loci within the H-2 complex that were most important for development of this response (Table 1). Significant DMBA contact hypersensitivity developed in B10.BR, B10.A(R1), and B10.A mice, each of which express the Kk haplotype. It was not observed in any of the other B10 congenic mice that did not express the Kk allele (B10, B10.S, B10.D2, B10.Q, B10.TL, or B10.AQR). Strains that expressed the Ik and/or the Sk haplotype without the Kk haplotype (B10.TL and B10.AQR) did not develop DMBA contact hypersensitivity. Taken together, the results indicate that class I rather than the class II genes were primarily responsible for the development of DMBA contact hypersensitivity.
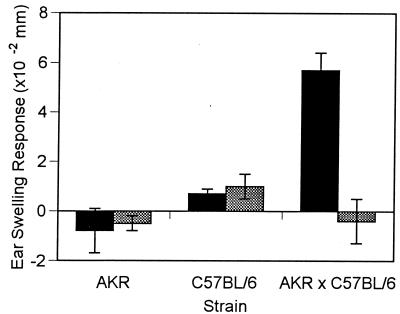
To further evaluate the role of the Ah receptor and the MHC in DMBA contact hypersensitivity, gene complementation studies were performed. The DMBA contact-hypersensitivity response in AKR × C57BL/10 F1 hybrids was compared with that of the two parental strains (Fig. 2). AKR mice possess the H-2k responding haplotype but carry the Ahd allele, which is associated with an inability to metabolize DMBA and therefore with an inability to develop DMBA contact hypersensitivity. C57BL/10 mice possess the nonresponding H-2b haplotype but carry the Ahb allele, which is necessary for metabolism of DMBA and has a permissive influence on the development of DMBA contact hypersensitivity. AKR × C57BL/10 F1 hybrids contain at least one responding allele at both genetic loci. Although neither parental haplotype was able to develop DMBA contact hypersensitivity, the F1 hybrids did develop a significant reaction (Fig. 2). These results provide strong evidence that both the appropriate Ah and MHC genetic loci are necessary for the development of contact hypersensitivity to carcinogenic PAHs.
Figure 2.
DMBA contact hypersensitivity response in AKR, C57BL/6, and AKRxC57BL/6 F1 hybrid mice. Panels of mice that were sensitized to 100 μl ofDMBA and were ear-challenged with 20 μl of DMBA served as positive controls (solid bars). Panels of mice that were only ear-challenged with 20 μl of DMBA served as negative controls (shaded bars).
MHC Influences on the Carcinogenic Activity of DMBA.
Although genes within the MHC influence the growth and metastatic behavior of fully developed tumors, there is no information available about MHC influences on earlier stages of the carcinogenesis pathway before the development of clinically apparent tumors. Because DMBA is carcinogenic, experiments were conducted to evaluate the influence of the MHC on the initiation stage of DMBA skin tumorigenesis. For this purpose, C3H/HeN and C3H.SW mice were given a single initiating dose of DMBA equivalent to that which was employed for induction of contact hypersensitivity. This was followed by biweekly treatments with the tumor promoter TPA. Tumor development was evaluated weekly. C3H/HeN and C3H.SW mice were employed for these experiments because they exhibited the greatest difference in contact hypersensitivity. It should also be noted that C57BL/10 mice are resistant to tumor promoters such as TPA, which makes tumorigenesis studies in this strain more difficult (1, 6). C3H/HeN mice (H-2k), which were found to exhibit a robust cell-mediated immune response to DMBA, developed significantly fewer tumors than C3H.SW mice (H-2d), which failed to do so (Fig. 3). This effect was also observed when the data were analyzed as the number of tumors per mouse, the percentage of mice with tumors, and the number of tumors per tumor-bearing mouse (Table 2).
Figure 3.
Cutaneous tumorigenesis in C3H and C3H.SW mice. C3H and C3H.SW mice were subjected to a DMBA-initiation, TPA-promotion cutaneous carcinogenesis protocol. The cumulative number of tumors was plotted as a function of the number of weeks on the test.
Table 2.
Tumor characteristics of DMBA-treated C3H mice
| Strain | Tumors/mouse | Percent of mice with tumors | Tumors/tumor-bearing mouse |
|---|---|---|---|
| C3H/HeN | 1.5 | 56 | 3.2 |
| C3H.SW | 4.2 | 85 | 4.6 |
Evaluations were performed at 25 weeks. P < 0.05 for all values.
To exclude the possibility that the difference in the carcinogenic activity in these two strains was the result of a difference in their reactivity to TPA, C3H/HeN and C3H.SW mice were treated with TPA. Ornithine decarboxylase activity and ear-swelling (a biological marker of inflammation) are known to increase after TPA treatment. There was no difference in the induction of ornithine decarboxylase activity or in ear swelling when the two strains were compared (Table 3), indicating that the difference in carcinogenic activity in these two strains was not caused by differential effects in the response to TPA.
Table 3.
Ornithine decarboxylase activity and ear swelling in TPA-treated C3H mice
| Hours after treatment | ODC activity
|
Ear swelling
|
||
|---|---|---|---|---|
| C3H/HeN | C3H.SW | C3H/HeN | C3H.SW | |
| 0 | 78 ± 9 | 72 ± 4 | ||
| 6 | 1824 ± 174 | 1732 ± 139 | 33.8 ± 3.3 | 37.8 ± 3.0 |
| 24 | 82 ± 12 | 79 ± 11 | 35.9 ± 3.8 | 40.6 ± 3.5 |
Four animals in each group were treated topically with TPA (40 nmol) and ODC activity was determined in epidermal cytosol as described in Materials and Methods. Data represent four independent values from each assay, conducted in triplicate. Four animals in each group were treated topically with TPA (32.4 nmol) and ear swelling was determined by measuring ear swelling in triplicate as described in Materials and Methods. The data are one of two representative experiments. Values given are mean ± SEM. None of the values in this table is statistically significant.
MHC Influences on the Formation of DMBA–DNA Adducts.
PAHs form adducts with DNA. There is a close correlation between adduct formation and the mutagenic and tumorigenic activity of PAHs (17). Experiments were performed to evaluate whether MHC differences played a role in the number of PAH–DNA adducts that were present in treated skin. To do this, the skin of C3H/HeN and C3H.SW mice was treated with carcinogenic doses of [3H]DMBA. At various times thereafter, the skin was removed, DNA was extracted, and the number of DMBA–DNA adducts was assessed. There were fewer adducts in skin removed from mice 24 hr after topical application of [3H]DMBA in C3H/HeN mice compared with C3H.SW mice (Table 4). After 10 days there was an increase in the disparity.
Table 4.
DMBA–DNA adducts in DMBA-treated C3H mice
| Days after [3H]- DMBA treatment | Covalent binding, pmol/mg DNA
|
Percent fewer DMBA–DNA adducts in C3H.SW mice | |
|---|---|---|---|
| C3H/HeN | C3H.SW | ||
| 1 | 117.2 ± 2.4 | 145.5 ± 2.0 | 19* |
| 10 | 8.6 ± 0.4 | 16.9 ± 0.6 | 49* |
[3H]DMBA was applied topically to the skin of mice. On the indicated day, the skin was removed and the covalent binding of [3H]DMBA to DNA was determined. ∗, significantly different at the P < 0.01 level.
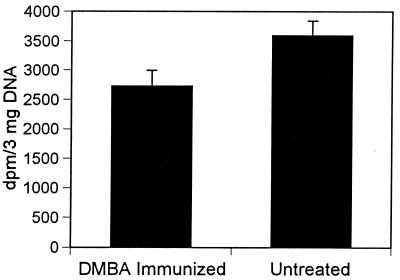
Studies were also conducted to determine whether in C3H mice, contact sensitization to DMBA was associated with a reduction in the number of DMBA–DNA adducts. C3H/HeN mice were sensitized to DMBA by painting 100 μl of a 0.1% solution to the shaved abdominal skin (positive control) or were left untreated (negative control). [3H]DMBA was applied to the back skin after 5 days, and skin was taken from the back for quantitation of DMBA–DNA adduct formation 24 hr later. Significantly fewer adducts were present in mice that had been presensitized to DMBA than in those that had not (Fig. 4).
Figure 4.
Effect of preimmunization with DMBA on subsequent DMBA–DNA adduct formation in the skin of C3H mice. Mice were immunized with DMBA by painting 100 μg on the abdominal skin. [3H]DMBA was applied to the back skin 5 days later. Skin was removed and [3H]DMBA binding to DNA was assessed 24 hr after that. Positive controls were immunized with DMBA before [3H]DMBA application. Negative controls were not immunized. The data are significant at the P < 0.01 level.
DISCUSSION
Normal cells only become malignant after they have progressed through a series of transitional, premalignant stages that occur over long periods of time (1, 18). In the first of these stages, called the tumor-initiation stage, chemical carcinogens produce mutations in the DNA of target cells. This alone is insufficient for cells to become fully malignant. During the second, or promotion, stage of carcinogenesis, repeated administration of tumor promoters produces additional biochemical changes in initiated cells that eventuate in the generation of premalignant papillomas. Additional genetic changes occur in a small percentage of the papillomas in the third, or progression, stage of carcinogenesis. These changes allow premalignant papillomas to become invasive carcinomas.
There has been great interest in identifying those genetic factors that increase susceptibility and resistance at each of these different stages. One of the genetic loci that predispose to the development of PAH-induced cancer is the Ah locus (5). The Ah locus encodes the intracellular receptor that is necessary for enzymatic conversion of the parent compound to a diol, epoxide, which is the actual carcinogenic moiety. Several other genetic factors that act during the promotion stage of carcinogenesis have been identified and are now being characterized.
It is generally acknowledged that a vigorous antitumor immune response exists to chemically induced tumors that have progressed through the carcinogenesis pathway. These malignancies express on their cell surface antigens that are recognized by immunocompetent lymphocytes (19, 20). Immunization techniques against tumor antigens have been successful in protecting animals against the subsequent growth of tumors that express the same antigens, findings which support the immunosurveillance theory that was originally proposed by Thomas (21) and Burnet (22). Vaccination procedures based on this information are currently undergoing clinical trials for selected human malignancies (23, 24).
In contrast to what is known about immune responses to clinically apparent tumors, there is comparatively little information on the host response at which the immune system interacts with the earlier stages in carcinogenesis. To investigate this issue, we examined several biological effects of topical PAH exposure in various strains of MHC congenic mice. In previous studies, we observed that topical application of PAHs results in the development of cell-mediated immunity to these agents (7, 8). Our studies indicate that this response cosegregates with genes of the MHC. H-2k and H-2a mice develop a contact hypersensitivity to DMBA, whereas H-2b, H-2d, and H-2s mice do not. These genetic loci were found also to impart resistance to the development of skin cancer by these agents. Furthermore, DMBA–DNA adducts, which reflect the initial interaction of chemical carcinogens with the host, were removed more rapidly in strains of mice that expressed MHC haplotypes associated with development of a cell-mediated immune response. These results are consistent with the hypothesis that the development of a cell-mediated immune response serves to protect the individual from the development of skin cancer by removing mutant cells before they can evolve into malignant neoplasms; these data would provide evidence for host defense mechanisms at a very early stage in the carcinogenesis pathway. Moreover, it would suggest that one of the important determinants of individual susceptibility to PAH-induced skin cancer is the inability to mount an immune response against the chemical carcinogen. Other evidence in support of this concept derives from the studies of Stutman (25), in which it was observed that the immunosuppressive effects of carcinogens were absent in mice that were resistant to chemical carcinogenesis protocols and were accelerated in those that did exhibit carcinogen-induced immune suppression. Recent observations by Svane and coworkers (26, 27) are also compatible with this hypothesis. They have shown that 3-methylcholanthrene-induced tumors derived from scid mice are more immunogenic than those produced in normal mice. The observation that prolonged administration of IL-12, which resulted in high levels of interferon γ production by CD8+ T cells and a shift in CD4+ T cells from a TH2 to a TH1 cytokine profile, delayed the appearance of tumors in animals treated with 3-methylcholanthrene lends further support to this concept (28).
An alternative method with which to examine the issue of the importance of a cell-mediated immunity in PAH carcinogenesis would be to induce tolerance to the PAH and then subject the tolerant animals to a DMBA tumorigenesis protocol. Unfortunately, we have been unable to induce antigen-specific immunosuppression to this compound, thereby preventing us from conducting this type of experiment. The reason that it is so hard to induce tolerance to DMBA is unclear, although it may relate to the metabolic pathway that this compound takes within the cell.
This study may have important implications for our understanding of genetic susceptibility to contact allergens. Most other agents, such as dinitrofluorobenzene, trinitrochlorobenzene, oxazolone, and fluorescein isothiocyanate, that have been used to analyze contact hypersensitivity in mice produce a response in virtually all strains of mice. With respect to DMBA, the inheritance patterns are quite clear and require genes encoding the appropriate set of class I MHC and Ah receptor proteins. Previous attempts to identify HLA associations with respect to contact allergens in humans have largely been unsuccessful, although an association of allergic contact dermatitis to nickel and the human MHC allele TaqI HLA–DQA allelic restriction fragment has been described (29).
If the theory that an immune response to DMBA-modified cells plays a protective role is borne out, then efforts to augment such a reaction may prove to be an effective preventive measure in individuals with increased risk for development of such tumors. Strategies could be devised in which noncarcinogenic analogues of PAHs are employed for immunization. Additionally, it may be possible to concomitantly administer adjuvants or cytokines with these analogues so as to augment their immunogenic potential. Alternatively, the risk of PAH-induced cancer may be assessed by determining his or her ability to metabolize PAHs (30) and by identifying his or her HLA type.
Acknowledgments
We appreciate the secretarial assistance of Laurie Adams. This work was supported by National Institutes of Health Grants AR39750, CA57643, CA48735, CA73096, and CA51802.
ABBREVIATIONS
- DMBA
dimethylbenz(a)anthracene
- MHC
major histocompatibility complex
- PAH
polyaromatic hydrocarbon
- TPA
phorbol 12-tetradecanoate 13-acetate
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.DiGiovanni J. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez F. In: Skin Cancer: Mechanisms and Human Relevance. Mukhtar H, editor. Boca Raton, FL: CRC; 1995. pp. 89–97. [Google Scholar]
- 3.Pelkonen O, Nebert D. Pharmacol Rev. 1982;34:189–222. [PubMed] [Google Scholar]
- 4.Quintanilla M, Brown K, Ramsden M, Balmain A. Nature (London) 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- 5.Conney A H. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 6.DiGiovanni J. Prog Clin Biol Res. 1995;391:195–212. [PubMed] [Google Scholar]
- 7.Klemme J, Mukhtar H, Elmets C. Cancer Res. 1987;47:6074–6078. [PubMed] [Google Scholar]
- 8.Anderson C, Hehr A, Robbins R, Hasan R, Athar M, Mukhtar H, Elmets C A. J Immunol. 1995;155:3530–3537. [PubMed] [Google Scholar]
- 9.Luster M, Rosenthal G. Environ Health Perspect. 1993;100:219–226. doi: 10.1289/ehp.93100219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruby J, Halliday G, Muller H. J Invest Dermatol. 1989;92:150–155. doi: 10.1111/1523-1747.ep12276661. [DOI] [PubMed] [Google Scholar]
- 11.Old L, Benacerraf B, Carswell E. Nature (London) 1963;198:1215–1216. doi: 10.1038/1981215a0. [DOI] [PubMed] [Google Scholar]
- 12.Pomeranz J. J Natl Cancer Inst. 1972;48:1513–1517. [PubMed] [Google Scholar]
- 13.Pomeranz J, Carney J, Alarif A. J Invest Dermatol. 1980;75:488–490. doi: 10.1111/1523-1747.ep12524261. [DOI] [PubMed] [Google Scholar]
- 14.Mukhtar H, Das M, Bickers D. Cancer Lett. 1986;31:147–151. doi: 10.1016/0304-3835(86)90005-4. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, DiIulio N, Fairchild R. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streilein J, Bergstresser P R. Immunogenetics. 1988;27:252–258. doi: 10.1007/BF00376119. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Katiyar S, Zaidi S, Mukhtar H. Cancer Res. 1992;52:3582–3588. [PubMed] [Google Scholar]
- 18.Mukhtar H, Mercurio M, Agarwal R. In: Skin Cancer: Mechanisms and Human Relevance. Mukhtar H, editor. Boca Raton, FL: CRC; 1995. pp. 3–8. [Google Scholar]
- 19.Pellis N, Kahan B. J Immunol. 1975;115:1717–1722. [PubMed] [Google Scholar]
- 20.Leffell M, Coggin J J. Cancer Res. 1977;37:4112–4119. [PubMed] [Google Scholar]
- 21.Thomas L. In: Cellular and Humoral Aspects of the Hypersensitive States. Lawrence H S, editor. New York: Hoeber-Harper; 1959. p. 529. [Google Scholar]
- 22.Burnet F. Immunological Surveillance. Oxford: Pergamon; 1970. [Google Scholar]
- 23.Conforti A, Ollila D, Kelley M, Gammon G, Morton D. J Surg Oncol. 1997;66:55–64. doi: 10.1002/(sici)1096-9098(199709)66:1<55::aid-jso12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.Wallack M, Sivanandham M, Ditaranto K, Shaw P, Balch C, Urist M, Bland K, Murray D, Robinson W, Flaherty L, et al. Ann Surg. 1997;226:198–206. doi: 10.1097/00000658-199708000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stutman O. Science. 1969;166:620–621. doi: 10.1126/science.166.3905.620. [DOI] [PubMed] [Google Scholar]
- 26.Engel A, Svane I, Rygaard J, Werdelin O. Scand J Immunol. 1997;45:463–470. doi: 10.1046/j.1365-3083.1997.d01-419.x. [DOI] [PubMed] [Google Scholar]
- 27.Svane I, Engel A, Nielsen M, Ljunggren H, Rygaard J, Werdelin O. Eur J Immunol. 1996;26:1844–1850. doi: 10.1002/eji.1830260827. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi Y, Jungbluth A, Richards E, Old L. Proc Natl Acad Sci USA. 1996;93:11798–11801. doi: 10.1073/pnas.93.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olerup O, Emtestam L. Immunogenetics. 1988;28:310–313. doi: 10.1007/BF00364228. [DOI] [PubMed] [Google Scholar]
- 30.Hirvonen A, Hersgafvel-Pursiainen K, Antilla S, Karjalainen A, Vainio H. Environ Health Perspect. 1993;101:109–112. doi: 10.1289/ehp.93101s3109. [DOI] [PMC free article] [PubMed] [Google Scholar]