Abstract
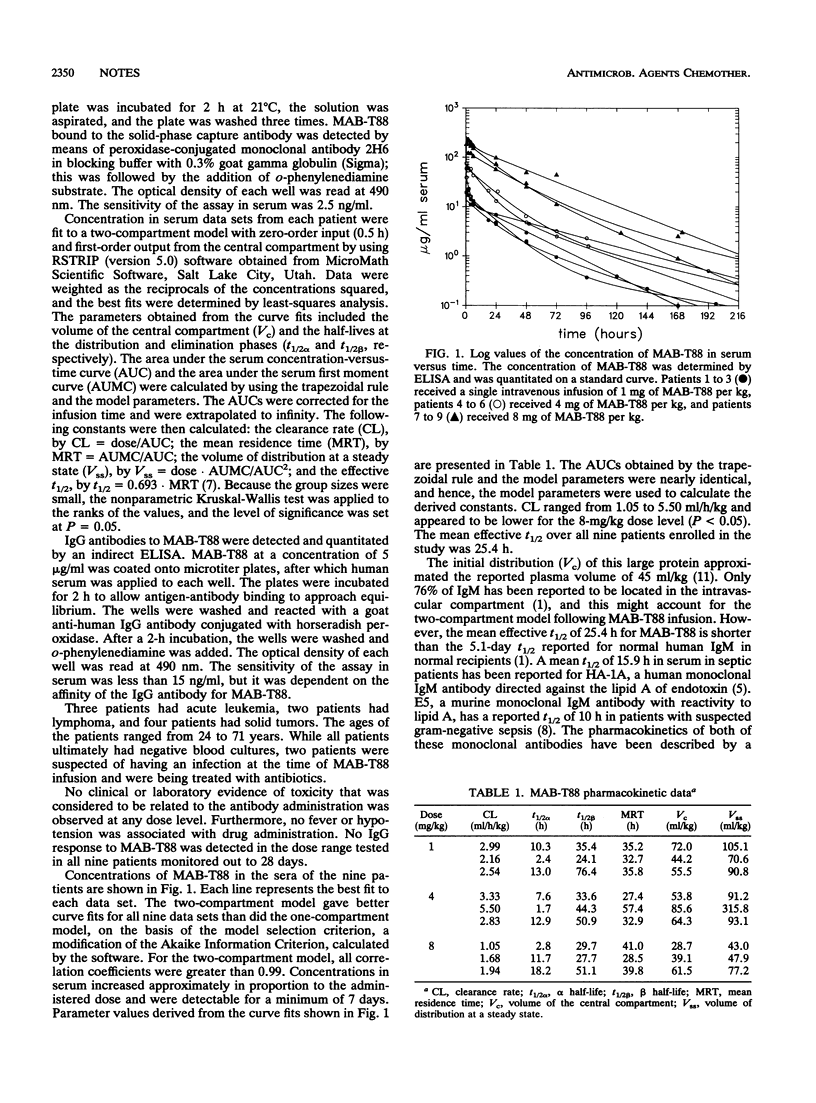
Monoclonal antibody MAB-T88 is a human monoclonal immunoglobulin M antibody directed at the lipopolysaccharide of gram-negative bacteria. In this study, nine patients who were expected to become neutropenic from antineoplastic chemotherapy received an infusion of MAB-T88, three patients at each of three doses: 1, 4, and 8 mg/kg of body weight. MAB-T88 was shown to be safe, with an effective half-life in plasma of 25.4 h, and no patient developed immunoglobulin G antibody to MAB-T88.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTH W. F., WOCHNER R. D., WALDMANN T. A., FAHEY J. L. METABOLISM OF HUMAN GAMMA MACROGLOBULINS. J Clin Invest. 1964 Jun;43:1036–1048. doi: 10.1172/JCI104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner J. D., Glauser M. P., McCutchan J. A., Ziegler E. J., van Melle G., Klauber M. R., Vogt M., Muehlen E., Luethy R., Chiolero R. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985 Jul 13;2(8446):59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Bussel J. B., Cunningham-Rundles C. Intravenous usage of gammaglobulin: humoral immunodeficiency, immune thrombocytopenic purpura, and newer indications. Cancer Invest. 1985;3(4):361–366. doi: 10.3109/07357908509039797. [DOI] [PubMed] [Google Scholar]
- Fisher C. J., Jr, Zimmerman J., Khazaeli M. B., Albertson T. E., Dellinger R. P., Panacek E. A., Foulke G. E., Dating C., Smith C. R., LoBuglio A. F. Initial evaluation of human monoclonal anti-lipid A antibody (HA-1A) in patients with sepsis syndrome. Crit Care Med. 1990 Dec;18(12):1311–1315. doi: 10.1097/00003246-199012000-00001. [DOI] [PubMed] [Google Scholar]
- Fomsgaard A., Baek L., Fomsgaard J. S., Engquist A. Preliminary study on treatment of septic shock patients with antilipopolysaccharide IgG from blood donors. Scand J Infect Dis. 1989;21(6):697–708. doi: 10.3109/00365548909021700. [DOI] [PubMed] [Google Scholar]
- Harkonen S., Scannon P., Mischak R. P., Spitler L. E., Foxall C., Kennedy D., Greenberg R. Phase I study of a murine monoclonal anti-lipid A antibody in bacteremic and nonbacteremic patients. Antimicrob Agents Chemother. 1988 May;32(5):710–716. doi: 10.1128/aac.32.5.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner G. H., Calabresi P. Selective suppression of humoral immunity by antineoplastic drugs. Annu Rev Pharmacol Toxicol. 1976;16:367–379. doi: 10.1146/annurev.pa.16.040176.002055. [DOI] [PubMed] [Google Scholar]
- KELLY K. H., BIERMAN H. R., SHIMKIN M. B. Blood volume, body water, and circulation time in patients with advanced neoplastic diseases. Cancer Res. 1952 Nov;12(11):814–817. [PubMed] [Google Scholar]
- Khazaeli M. B., Wheeler R., Rogers K., Teng N., Ziegler E., Haynes A., Saleh M. N., Hardin J. M., Bolmer S., Cornett J. Initial evaluation of a human immunoglobulin M monoclonal antibody (HA-1A) in humans. J Biol Response Mod. 1990 Apr;9(2):178–184. [PubMed] [Google Scholar]
- Kistler D., Piert M., Kauhl W., Hettich R. Use of Pseudomonas hyperimmunoglobulin to treat septic shock in burn cases. J Burn Care Rehabil. 1989 Jul-Aug;10(4):321–326. doi: 10.1097/00004630-198907000-00006. [DOI] [PubMed] [Google Scholar]
- Lachman E., Pitsoe S. B., Gaffin S. L. Anti-lipopolysaccharide immunotherapy in management of septic shock of obstetric and gynaecological origin. Lancet. 1984 May 5;1(8384):981–983. doi: 10.1016/s0140-6736(84)92324-9. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Parker M. M., Natanson C., Suffredini A. F., Danner R. L., Cunnion R. E., Ognibene F. P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990 Aug 1;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Lam K. S., Calvo Riera F., Kaplan H. S. Construction and testing of mouse--human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7308–7312. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L., Gauny S. S., Senyk G., Piazza D., Stevens P. Human monoclonal antibodies to glycolipid A that exhibit complement species-specific effector functions. J Infect Dis. 1992 Jan;165(1):26–33. doi: 10.1093/infdis/165.1.26. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]


