Abstract
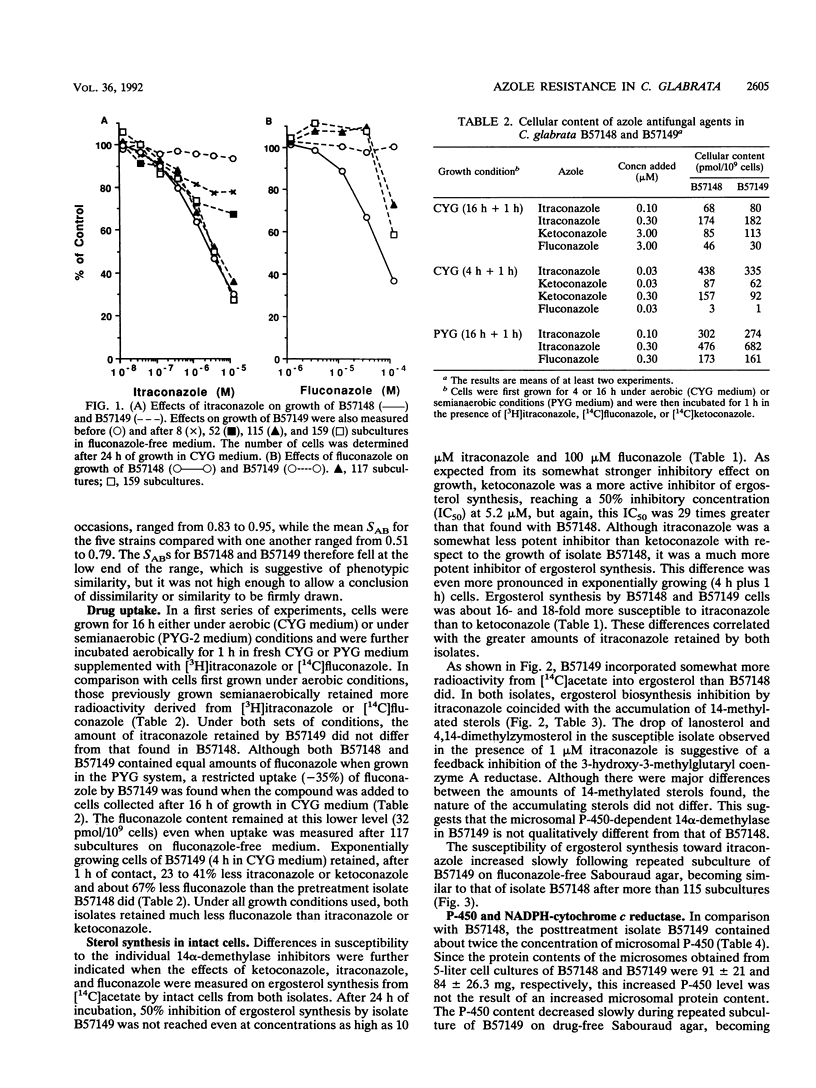
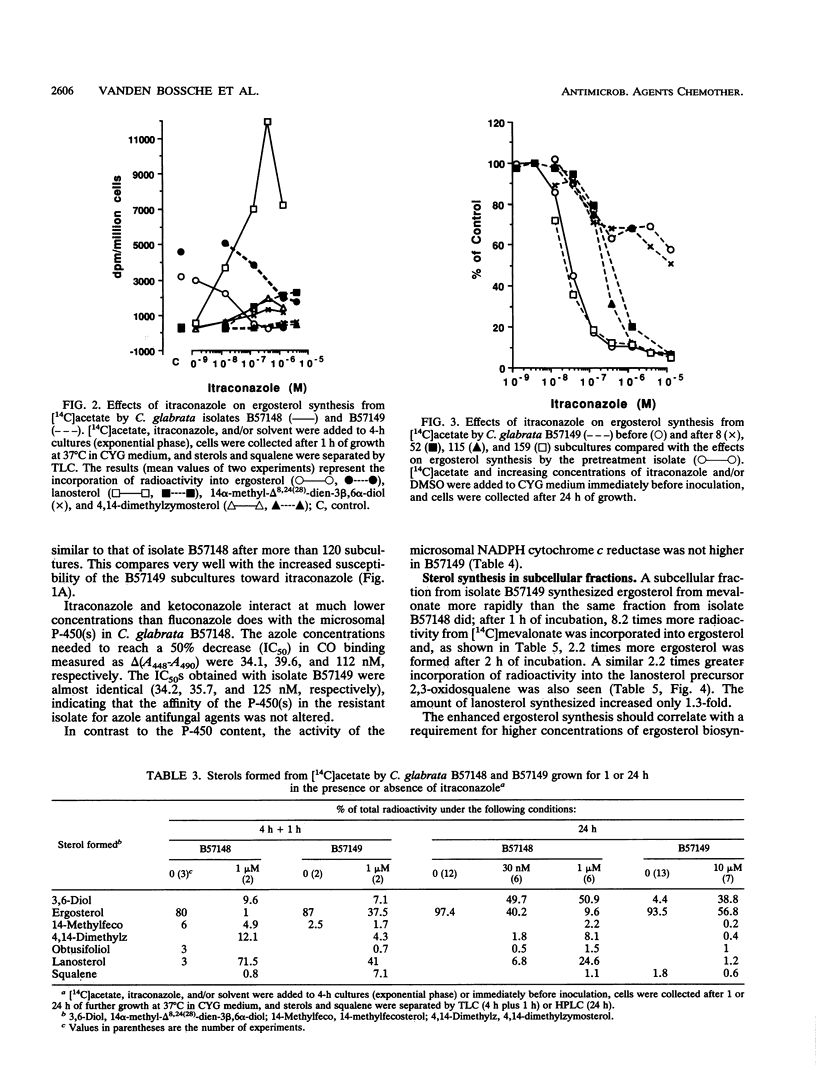
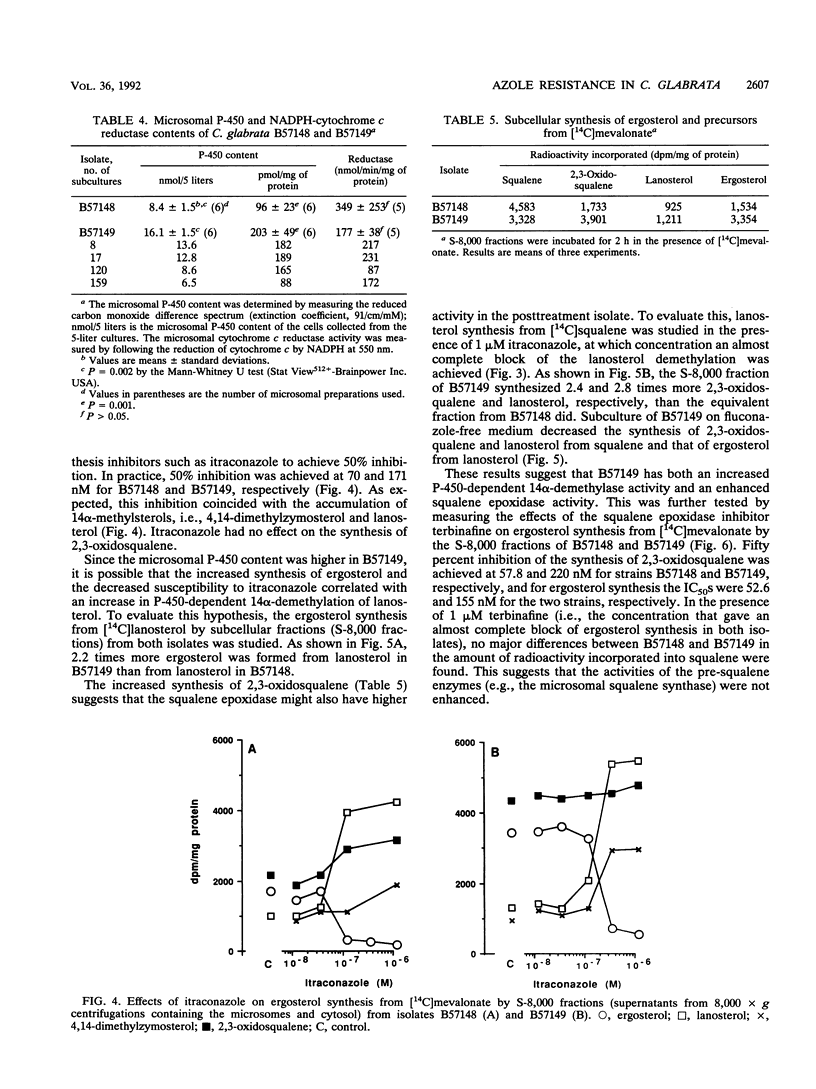
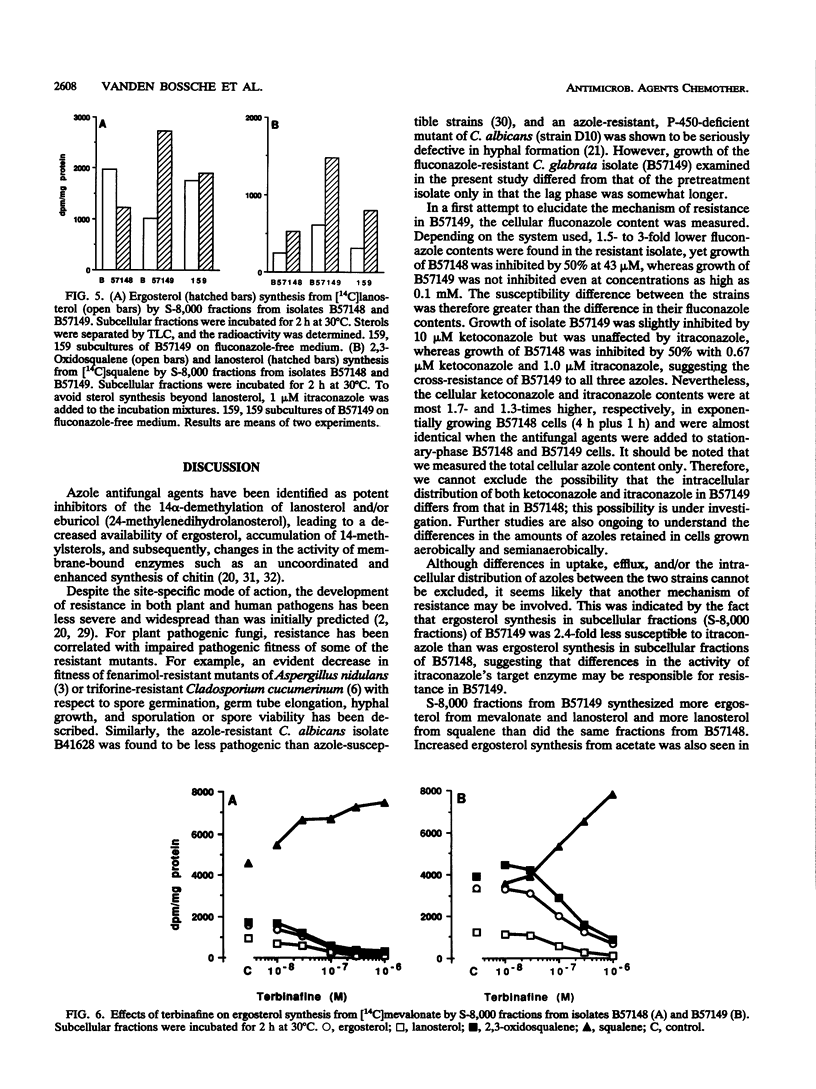
A Candida (Torulopsis) glabrata strain (B57149) became resistant to fluconazole after a patient carrying the organism was treated with the drug at 400 mg once daily for 9 days. Growth of the pretreatment isolate (B57148) was inhibited by 50% with 0.67 microM ketoconazole, 1.0 microM itraconazole, and 43 microM fluconazole, whereas growth of B57149 was inhibited slightly by 10 microM ketoconazole but was unaffected by 10 microM itraconazole or 100 microM fluconazole. This indicates cross-resistance to all three azole antifungal agents. The cellular fluconazole content of B57149 was from 1.5- to 3-fold lower than that of B57148, suggesting a difference in drug uptake between the strains. However, this difference was smaller than the measured difference in susceptibility and, therefore, cannot fully explain the fluconazole resistance of B57149. Moreover, the intracellular contents of ketoconazole and itraconazole differed by less than twofold between the strains, so that uptake differences did not account for the azole cross-resistance of B57149. The microsomal cytochrome P-450 content of B57149 was about twice that of B57148, a difference quantitatively similar to the increased subcellular ergosterol synthesis from mevalonate or lanosterol. These results indicate that the level of P-450-dependent 14 alpha-demethylation of lanosterol is higher in B57149. Increased ergosterol synthesis was also seen in intact B57149 cells, and this coincided with a decreased susceptibility of B57149 toward all three azoles and amphotericin B. B57149 also had higher squalene epoxidase activity, and thus, more terbinafine was needed to inhibit the synthesis of 2,3-oxidosqualene from squalene. P-450 content and ergosterol synthesis both decreased when isolate B57149 was subcultured repeatedly on drug-free medium. This repeated subculture also fully restored the strain's itraconazole susceptibility, but only partly increased its susceptibility to fluconazole. The results suggest that both lower fluconazole uptake and increased P-450-dependent ergosterol synthesis are involved in the mechanism of fluconazole resistance but that only the increased ergosterol synthesis contributes to itraconazole cross-resistance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C., Turi T. G., Sanglard D., Loper J. C. Isolation of the Candida tropicalis gene for P450 lanosterol demethylase and its expression in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1311–1317. doi: 10.1016/0006-291x(87)90792-3. [DOI] [PubMed] [Google Scholar]
- Dupouy-Camet J., Paugam A., Di Donato C., Viguié C., Vicens I., Volle P. J., Tourte-Schaefer C. Résistance au fluconazole en milieu hospitalier. Concordance entre la résistance de Candida albicans in vitro et l'échec thérapeutique. Presse Med. 1991 Sep 14;20(28):1341–1341. [PubMed] [Google Scholar]
- Heykants J., Van Peer A., Van de Velde V., Van Rooy P., Meuldermans W., Lavrijsen K., Woestenborghs R., Van Cutsem J., Cauwenbergh G. The clinical pharmacokinetics of itraconazole: an overview. Mycoses. 1989;32 (Suppl 1):67–87. doi: 10.1111/j.1439-0507.1989.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. Inhibition of 14 alpha-sterol demethylase activity in Candida albicans Darlington does not correlate with resistance to azole. J Med Vet Mycol. 1987 Oct;25(5):329–333. [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J Gen Microbiol. 1986 Sep;132(9):2421–2431. doi: 10.1099/00221287-132-9-2421. [DOI] [PubMed] [Google Scholar]
- Holt R. J., Azmi A. Miconazole-resistant Candida. Lancet. 1978 Jan 7;1(8054):50–51. doi: 10.1016/s0140-6736(78)90403-8. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Kirkpatrick C. H. Long-term therapy of chronic mucocutaneous candidiasis with ketoconazole: experience with twenty-one patients. Am J Med. 1983 Jan 24;74(1B):23–29. doi: 10.1016/0002-9343(83)90511-9. [DOI] [PubMed] [Google Scholar]
- Howell S. A., Mallet A. I., Noble W. C. A comparison of the sterol content of multiple isolates of the Candida albicans Darlington strain with other clinically azole-sensitive and -resistant strains. J Appl Bacteriol. 1990 Nov;69(5):692–696. doi: 10.1111/j.1365-2672.1990.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Kalb V. F., Loper J. C., Dey C. R., Woods C. W., Sutter T. R. Isolation of a cytochrome P-450 structural gene from Saccharomyces cerevisiae. Gene. 1986;45(3):237–245. doi: 10.1016/0378-1119(86)90021-1. [DOI] [PubMed] [Google Scholar]
- Kelly S. L., Rowe J., Watson P. F. Molecular genetic studies on the mode of action of azole antifungal agents. Biochem Soc Trans. 1991 Aug;19(3):796–798. doi: 10.1042/bst0190796. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Fasoli M., Wayman F. J. Drug resistance in Candida albicans and Candida glabrata. Ann N Y Acad Sci. 1988;544:245–259. doi: 10.1111/j.1749-6632.1988.tb40410.x. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Nicholas R. O. Drug resistance in the opportunistic pathogens Candida albicans and Candida glabrata. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):39–49. doi: 10.1093/jac/18.supplement_b.39. [DOI] [PubMed] [Google Scholar]
- Kirsch D. R., Lai M. H., O'Sullivan J. Isolation of the gene for cytochrome P450L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Gene. 1988 Sep 7;68(2):229–237. doi: 10.1016/0378-1119(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Kitchen V. S., Savage M., Harris J. R. Candida albicans resistance in AIDS. J Infect. 1991 Mar;22(2):204–205. doi: 10.1016/0163-4453(91)91789-z. [DOI] [PubMed] [Google Scholar]
- Nicholas R. O., Burton J. A., Kerridge D., Wayman F. J. Isolation of mutants of Candida glabrata resistant to miconazole. Crit Rev Microbiol. 1987;15(1):103–110. doi: 10.3109/10408418709104453. [DOI] [PubMed] [Google Scholar]
- Nobre G., Mendes E., Charrua M. J., Cruz O. Ketoconazole resistance in Torulopsis glabrata. Mycopathologia. 1989 Jul;107(1):51–55. doi: 10.1007/BF00437589. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Quantitative microculture system with standardized inocula for strain typing, susceptibility testing, and other physiologic measurements with Candida albicans and other yeasts. J Clin Microbiol. 1991 Dec;29(12):2735–2740. doi: 10.1128/jcm.29.12.2735-2740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryley J. F., Wilson R. G., Barrett-Bee K. J. Azole resistance in Candida albicans. Sabouraudia. 1984;22(1):53–63. [PubMed] [Google Scholar]
- Schmid J., Voss E., Soll D. R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990 Jun;28(6):1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Warnock D. W., Kennedy C. T., Johnson E. M., Hopwood V., Van Cutsem J., Vanden Bossche H. Azole resistance in Candida albicans. J Med Vet Mycol. 1986 Apr;24(2):133–144. [PubMed] [Google Scholar]
- Vanden Bossche H. Biochemical targets for antifungal azole derivatives: hypothesis on the mode of action. Curr Top Med Mycol. 1985;1:313–351. doi: 10.1007/978-1-4613-9547-8_12. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Gorrens J., Bellens D., Moereels H., Janssen P. A. Mutation in cytochrome P-450-dependent 14 alpha-demethylase results in decreased affinity for azole antifungals. Biochem Soc Trans. 1990 Feb;18(1):56–59. doi: 10.1042/bst0180056. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Willemsens G., Bellens D., Gorrens J., Roels I., Coene M. C., Le Jeune L., Janssen P. A. Saperconazole: a selective inhibitor of the cytochrome P-450-dependent ergosterol synthesis in Candida albicans, Aspergillus fumigatus and Trichophyton mentagrophytes. Mycoses. 1990 Jul-Aug;33(7-8):335–352. doi: 10.1111/myc.1990.33.7-8.335. [DOI] [PubMed] [Google Scholar]
- Vermilion J. L., Coon M. J. Purified liver microsomal NADPH-cytochrome P-450 reductase. Spectral characterization of oxidation-reduction states. J Biol Chem. 1978 Apr 25;253(8):2694–2704. [PubMed] [Google Scholar]
- Warnock D. W., Burke J., Cope N. J., Johnson E. M., von Fraunhofer N. A., Williams E. W. Fluconazole resistance in Candida glabrata. Lancet. 1988 Dec 3;2(8623):1310–1310. doi: 10.1016/s0140-6736(88)92919-4. [DOI] [PubMed] [Google Scholar]
- Warnock D. W., Johnson E. M., Richardson M. D., Vickers C. F. Modified response to ketoconazole of Candida albicans from a treatment failure. Lancet. 1983 Mar 19;1(8325):642–643. doi: 10.1016/s0140-6736(83)91809-3. [DOI] [PubMed] [Google Scholar]
- Willocks L., Leen C. L., Brettle R. P., Urquhart D., Russell T. B., Milne L. J. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991 Dec;28(6):937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]
- van den Bossche H., Willemsens G., Cools W., Lauwers W. F., Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem Biol Interact. 1978 Apr;21(1):59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]


