Abstract
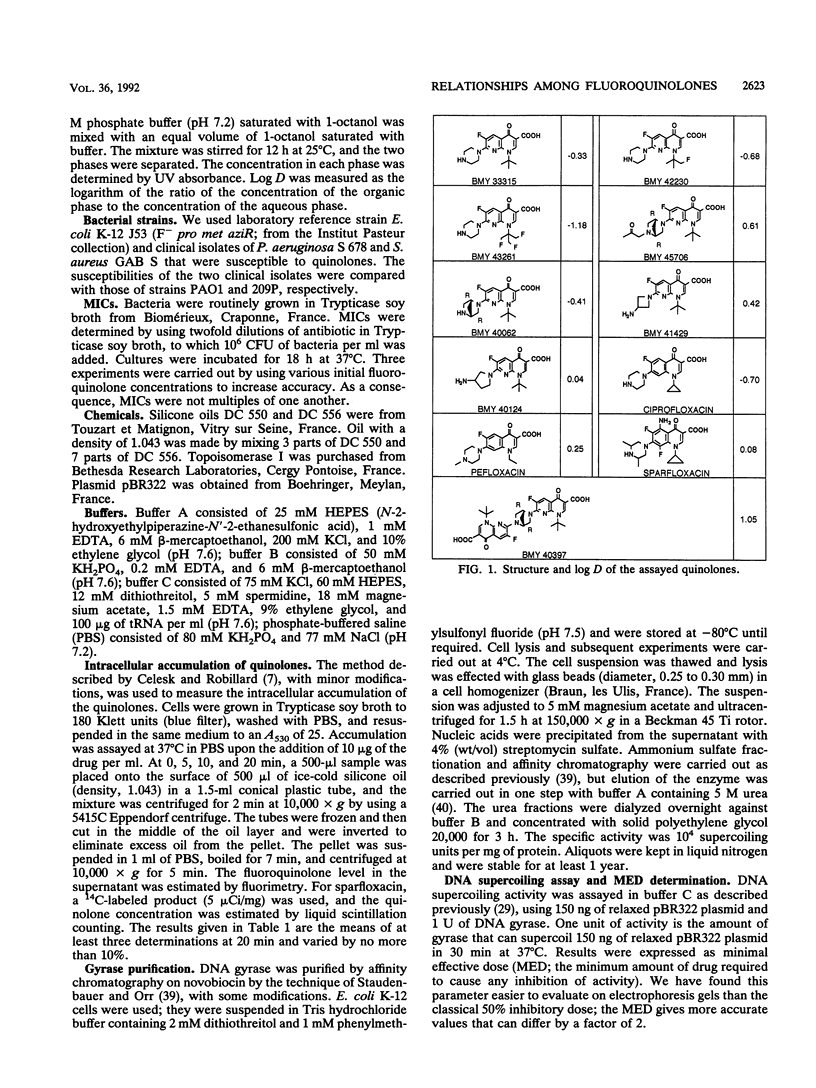
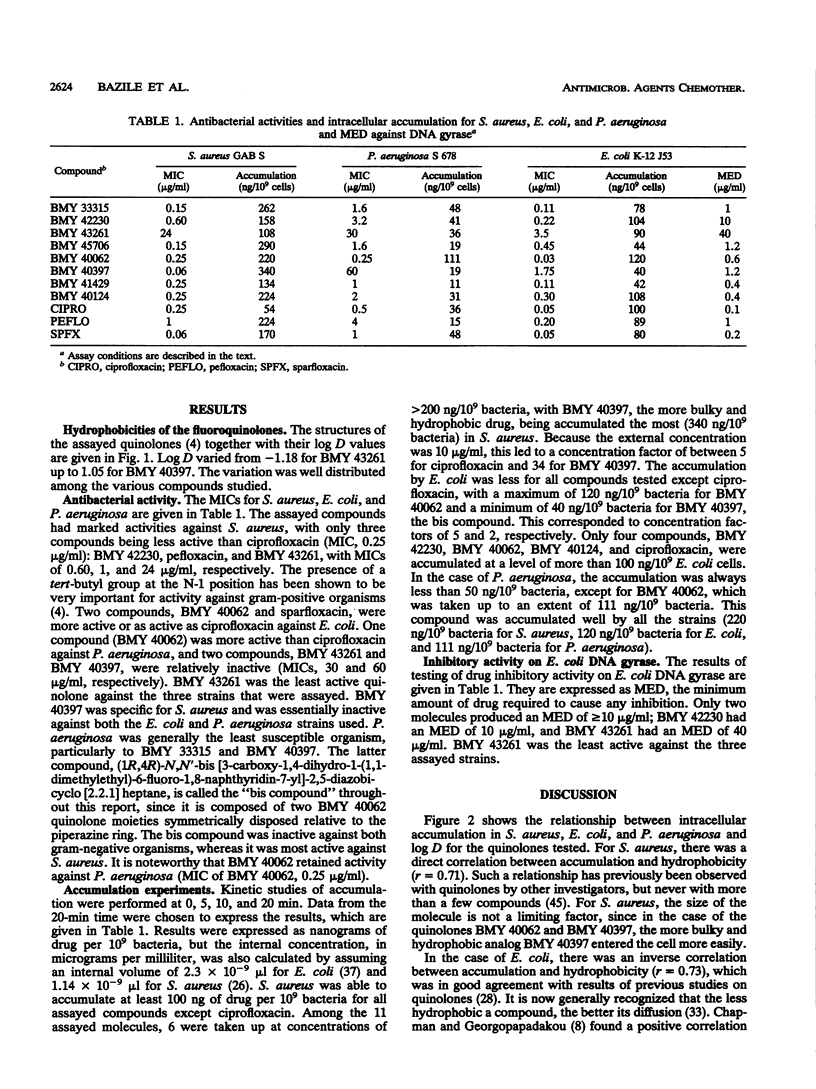
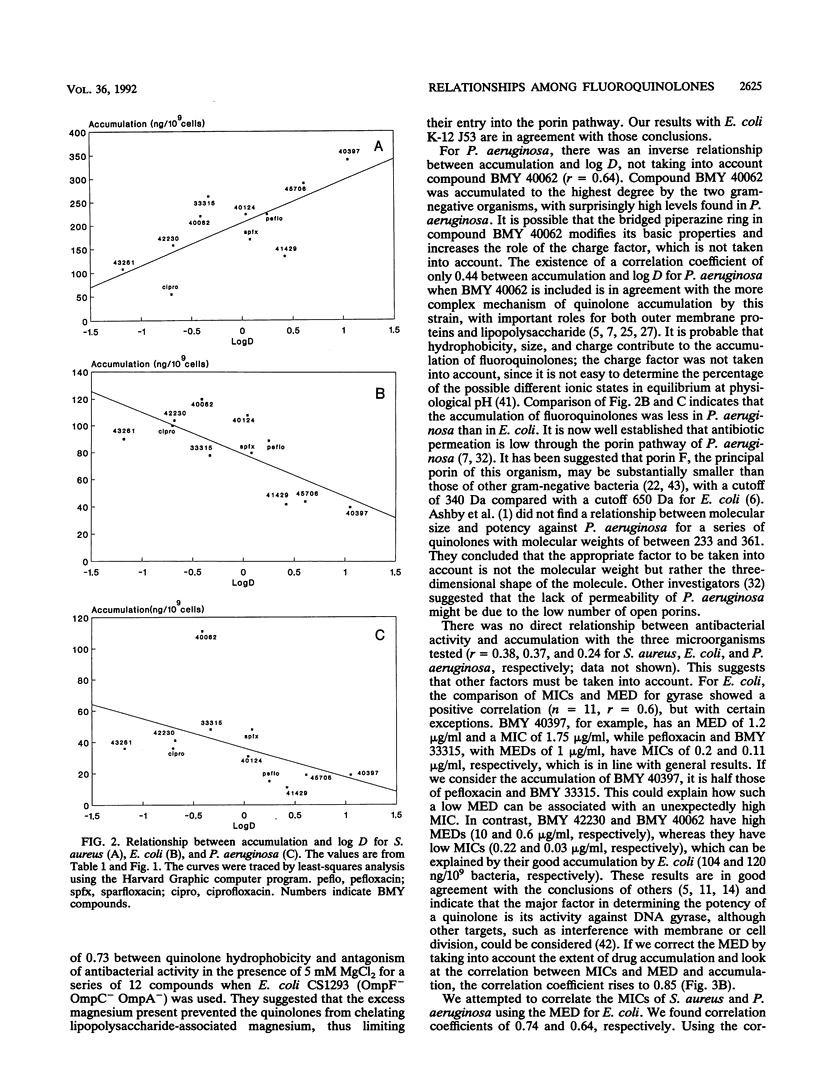
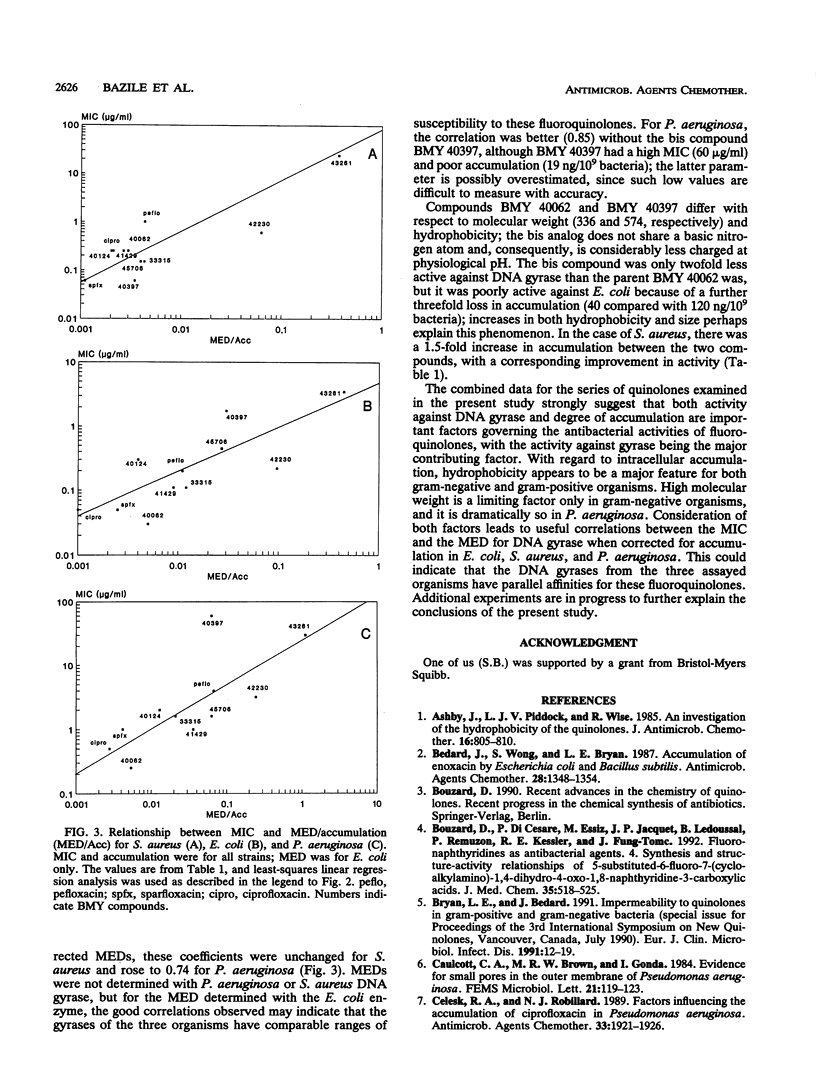
A series of 11 fluoroquinolone antibacterial agents, including 8 newly synthesized molecules and 3 reference compounds (pefloxacin, ciprofloxacin, and sparfloxacin), were tested for their MICs against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The intracellular accumulation of fluoroquinolones by these microorganisms was measured by centrifugation through silicone oil and a fluorescence assay. The minimal effective dose (MED) was determined for all agents in a supercoiling assay with E. coli DNA gyrase. The hydrophobicities of the quinolones were determined and expressed as the logarithm of the coefficient of distribution (log D) between 1-octanol and phosphate buffer (pH 7.2). No correlation was found between MICs and cell accumulation for the quinolones studied. A correlation was found between log D and accumulation by S. aureus (r = 0.71, n = 11), and an inverse correlation was found between log D and accumulation by E. coli (r = 0.73, n = 11) and P. aeruginosa (r = 0.64, n = 10). The correlation coefficients between MICs and MED for E. coli, which were 0.60, 0.64, and 0.74 (n = 11) for E. coli, P. aeruginosa, and S. aureus, respectively, rose to 0.85, 0.74, and 0.74 (n = 11) for the same microorganisms, respectively, when the accumulation of the drug by the cell was taken into account. It was concluded that the inhibitory activity against DNA gyrase remains the most important parameter for quinolone potency, but that intracellular accumulation must be taken into account, since, for a given organism, both parameters are under the control of the physicochemical properties of the quinolones.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Piddock L. J., Wise R. An investigation of the hydrophobicity of the quinolones. J Antimicrob Chemother. 1985 Dec;16(6):805–808. doi: 10.1093/jac/16.6.805-a. [DOI] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzard D., Di Cesare P., Essiz M., Jacquet J. P., Ledoussal B., Remuzon P., Kessler R. E., Fung-Tomc J. Fluoronaphthyridines as antibacterial agents. 4. Synthesis and structure-activity relationships of 5-substituted-6-fluoro-7-(cycloalkylamino)-1,4-dihydro-4-oxo-1,8- naphthyridine-3-carboxylic acids. J Med Chem. 1992 Feb 7;35(3):518–525. doi: 10.1021/jm00081a013. [DOI] [PubMed] [Google Scholar]
- Celesk R. A., Robillard N. J. Factors influencing the accumulation of ciprofloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Nov;33(11):1921–1926. doi: 10.1128/aac.33.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. T., Fernandes P. B. Structure-activity relationships of the fluoroquinolones. Antimicrob Agents Chemother. 1989 Feb;33(2):131–135. doi: 10.1128/aac.33.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver J. M., Piddock L. J., Wise R. The accumulation of five quinolone antibacterial agents by Escherichia coli. J Antimicrob Chemother. 1990 Mar;25(3):319–333. doi: 10.1093/jac/25.3.319. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Heifetz C. L., Hutt M. P., Mich T. F., Nichols J. B., Solomon M., Worth D. F. 1-Substituted 7-[3-[(ethylamino)methyl]-1-pyrrolidinyl]-6,8- difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acids. New quantitative structure-activity relationships at N1 for the quinolone antibacterials. J Med Chem. 1988 May;31(5):991–1001. doi: 10.1021/jm00400a017. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Role of porins in outer membrane permeability. J Bacteriol. 1987 Mar;169(3):929–933. doi: 10.1128/jb.169.3.929-933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Macina O. T., Levinson M. E., Rosenkranz H. S. Computer automated structure evaluation of quinolone antibacterial agents. Antimicrob Agents Chemother. 1987 Nov;31(11):1831–1840. doi: 10.1128/aac.31.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masecar B. L., Celesk R. A., Robillard N. J. Analysis of acquired ciprofloxacin resistance in a clinical strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Feb;34(2):281–286. doi: 10.1128/aac.34.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates S. M., Eisenberg E. S., Mandel L. J., Patel L., Kaback H. R., Miller M. H. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Furet Y. X., Pechère J. C. Role of protein D2 and lipopolysaccharide in diffusion of quinolones through the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991 Oct;35(10):2091–2097. doi: 10.1128/aac.35.10.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau N. J., Robaux H., Baron L., Tabary X. Inhibitory effects of quinolones on pro- and eucaryotic DNA topoisomerases I and II. Antimicrob Agents Chemother. 1990 Oct;34(10):1955–1960. doi: 10.1128/aac.34.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Kojima T., Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram M., Fisher L. M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991 Feb;35(2):387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen T. The fluoroquinolone antibacterial agents. Prog Med Chem. 1990;27:235–295. doi: 10.1016/s0079-6468(08)70293-5. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Sreedharan S., Oram M., Jensen B., Peterson L. R., Fisher L. M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabary X., Moreau N., Dureuil C., Le Goffic F. Effect of DNA gyrase inhibitors pefloxacin, five other quinolones, novobiocin, and clorobiocin on Escherichia coli topoisomerase I. Antimicrob Agents Chemother. 1987 Dec;31(12):1925–1928. doi: 10.1128/aac.31.12.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács-Novák K., Noszál B., Hermecz I., Keresztúri G., Podányi B., Szász G. Protonation equilibria of quinolone antibacterials. J Pharm Sci. 1990 Nov;79(11):1023–1028. doi: 10.1002/jps.2600791116. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., McHugh G. L., Bozza M. A., Swartz M. N. Mutants of Escherichia coli K-12 exhibiting reduced killing by both quinolone and beta-lactam antimicrobial agents. Antimicrob Agents Chemother. 1990 Oct;34(10):1938–1943. doi: 10.1128/aac.34.10.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Kojima T., Inoue M., Mitsuhashi S. Uptake of sparfloxacin and norfloxacin by clinical isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):368–370. doi: 10.1128/aac.35.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


