Abstract
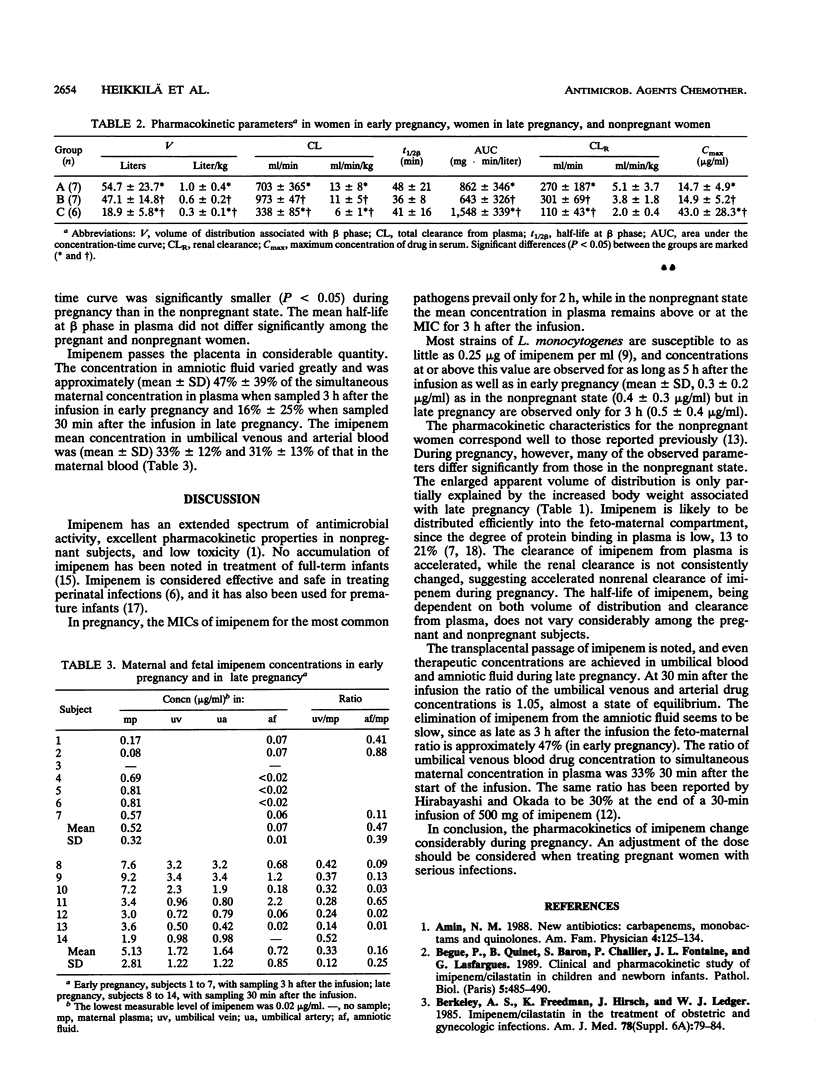
Imipenem pharmacokinetics were studied in early pregnancy (n = 7; length of gestation, 8.6 +/- 1.5 weeks, mean +/- standard deviation), in late pregnancy (n = 7; length of gestation, 38.7 +/- 1.4 weeks), and in the nonpregnant state (n = 6). A single dose of 500 mg of imipenem-cilastatin (1:1) was administered as a 20-min infusion. Multiple plasma and urine samples, as well as specimens of umbilical plasma and amniotic fluid from the pregnant subjects, were collected at frequent intervals for 8 h. Imipenem concentrations were assayed by specific microbiologic assay. The mean peak concentrations in plasma were 14.7 +/- 4.9, 14.9 +/- 5.2, and 43 +/- 28.3 micrograms/ml in early pregnancy, late pregnancy, and the nonpregnant state, respectively. The volumes of distribution were significantly larger during early pregnancy (0.98 +/- 0.45 liter/kg of body weight, P < 0.005) and late pregnancy (0.59 +/- 0.19 liter/kg, P < 0.05) than in the nonpregnant state (0.33 +/- 0.10 liter/kg), and total clearances from plasma were faster in early pregnancy (12.7 +/- 7.8 ml min-1 kg-1, P < 0.05) and late pregnancy (10.7 +/- 4.6 ml min-1 kg-1, P < 0.05) than in the nonpregnant state (5.77 +/- 1.19 ml min-1 kg-1). The mean concentrations in amniotic fluid were 0.07 +/- 0.01 and 0.72 +/- 0.85 micrograms/ml in early and late pregnancy. The mean umbilical venous and arterial drug concentrations were 1.72 +/- 1.22 and 1.64 +/- 1.22 micrograms/ml. The feto-maternal ratio at the time of cesarean section was 0.33 +/- 0.12. These results indicate that an adjustment of doses of imipenem may be required when treating pregnant women because of considerable changes in imipenem pharmacokinetics during pregnancy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin N. M. New antibiotics: carbapenems, monobactams and quinolones. Am Fam Physician. 1988 Oct;38(4):125–134. [PubMed] [Google Scholar]
- Berkeley A. S., Freedman K., Hirsch J., Ledger W. J. Imipenem/cilastatin in the treatment of obstetric and gynecologic infections. Am J Med. 1985 Jun 7;78(6A):79–84. doi: 10.1016/0002-9343(85)90105-6. [DOI] [PubMed] [Google Scholar]
- Birnbaum J., Kahan F. M., Kropp H., MacDonald J. S. Carbapenems, a new class of beta-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J Med. 1985 Jun 7;78(6A):3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Del Bene V. E., Collins C. D. In vitro activity of N-formimidoyl thienamycin, moxalactam, and other new beta-lactam agents against Bacteroides fragilis: contribution of beta-lactamase to resistance. Antimicrob Agents Chemother. 1981 Feb;19(2):248–252. doi: 10.1128/aac.19.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégué P., Quinet B., Baron S., Challier P., Fontaine J. L., Lasfargues G. Etude clinique et pharmacocinétique de l'imipénème/cilastatine chez l'enfant et le nouveau-né. Pathol Biol (Paris) 1989 May;37(5):485–490. [PubMed] [Google Scholar]
- Cho N., Fukunaga K., Kunii K., Kobayashi I., Tezuka K. [Studies on imipenem/cilastatin sodium in the perinatal period]. Jpn J Antibiot. 1988 Nov;41(11):1758–1773. [PubMed] [Google Scholar]
- Drusano G. L., Standiford H. C. Pharmacokinetic profile of imipenem/cilastatin in normal volunteers. Am J Med. 1985 Jun 7;78(6A):47–53. doi: 10.1016/0002-9343(85)90101-9. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Moellering R. C., Jr Susceptibility of enterococci and Listeria monocytogenes to N-Formimidoyl thienamycin alone and in combination with an aminoglycoside. Antimicrob Agents Chemother. 1981 May;19(5):789–793. doi: 10.1128/aac.19.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä A., Erkkola R. Pharmacokinetics of piperacillin during pregnancy. J Antimicrob Chemother. 1991 Sep;28(3):419–423. doi: 10.1093/jac/28.3.419. [DOI] [PubMed] [Google Scholar]
- Heikkilä A., Pyykkö K., Erkkola R., Iisalo E. The pharmacokinetics of mecillinam and pivmecillinam in pregnant and non-pregnant women. Br J Clin Pharmacol. 1992 Jun;33(6):629–633. doi: 10.1111/j.1365-2125.1992.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi K., Okada E. [Pharmacokinetic and clinical studies of imipenem/cilastatin sodium in the perinatal period]. Jpn J Antibiot. 1988 Nov;41(11):1797–1804. [PubMed] [Google Scholar]
- MacGregor R. R., Gibson G. A., Bland J. A. Imipenem pharmacokinetics and body fluid concentrations in patients receiving high-dose treatment for serious infections. Antimicrob Agents Chemother. 1986 Feb;29(2):188–192. doi: 10.1128/aac.29.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Suzuki M., Oh K., Ishikawa M., Soma A., Takada H., Shimizu T., Makinoda S., Fujimoto S., Chimura T. [Pharmacokinetic and clinical studies on imipenem/cilastatin sodium in the perinatal period. A study of imipenem/cilastatin sodium in the perinatal co-research group]. Jpn J Antibiot. 1988 Nov;41(11):1731–1741. [PubMed] [Google Scholar]
- Musson D. G., Hajdu R., Bayne W. F., Rogers J. D. Quantification of imipenem's primary metabolite in plasma by postcolumn chemical rearrangement and UV detection. Pharm Res. 1991 Jan;8(1):33–39. doi: 10.1023/a:1015818004113. [DOI] [PubMed] [Google Scholar]
- Reed M. D., Kliegman R. M., Yamashita T. S., Myers C. M., Blumer J. L. Clinical pharmacology of imipenem and cilastatin in premature infants during the first week of life. Antimicrob Agents Chemother. 1990 Jun;34(6):1172–1177. doi: 10.1128/aac.34.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F., Knott C. Pharmacokinetics in pregnancy and placental drug transfer. Oxf Rev Reprod Biol. 1989;11:389–449. [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. In vitro activity of N-formimidoyl thienamycin in comparison with cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Mar;19(3):402–406. doi: 10.1128/aac.19.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]


