Abstract
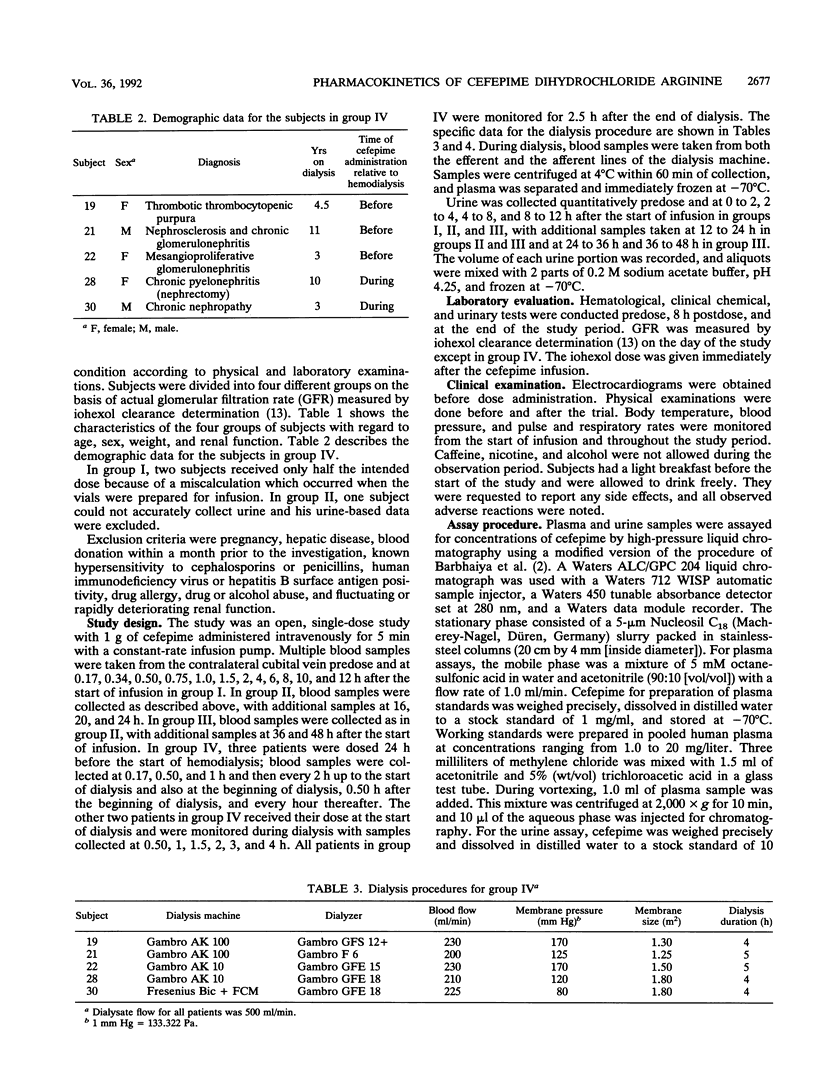
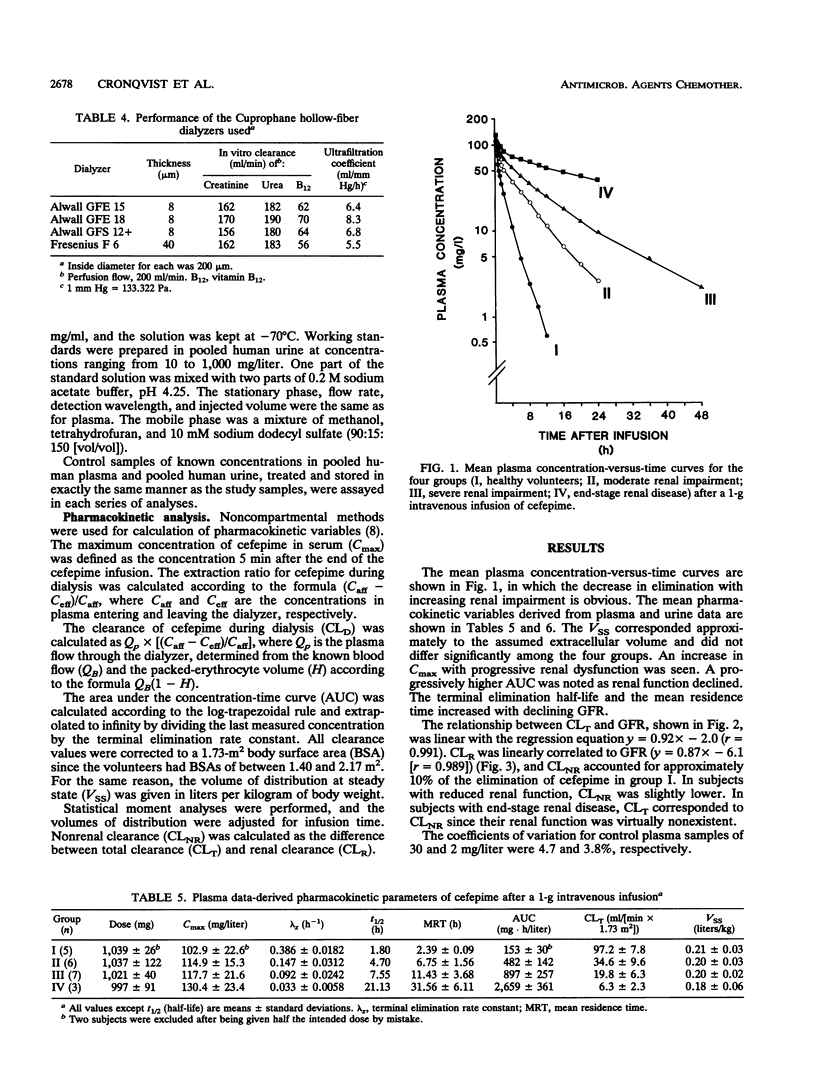
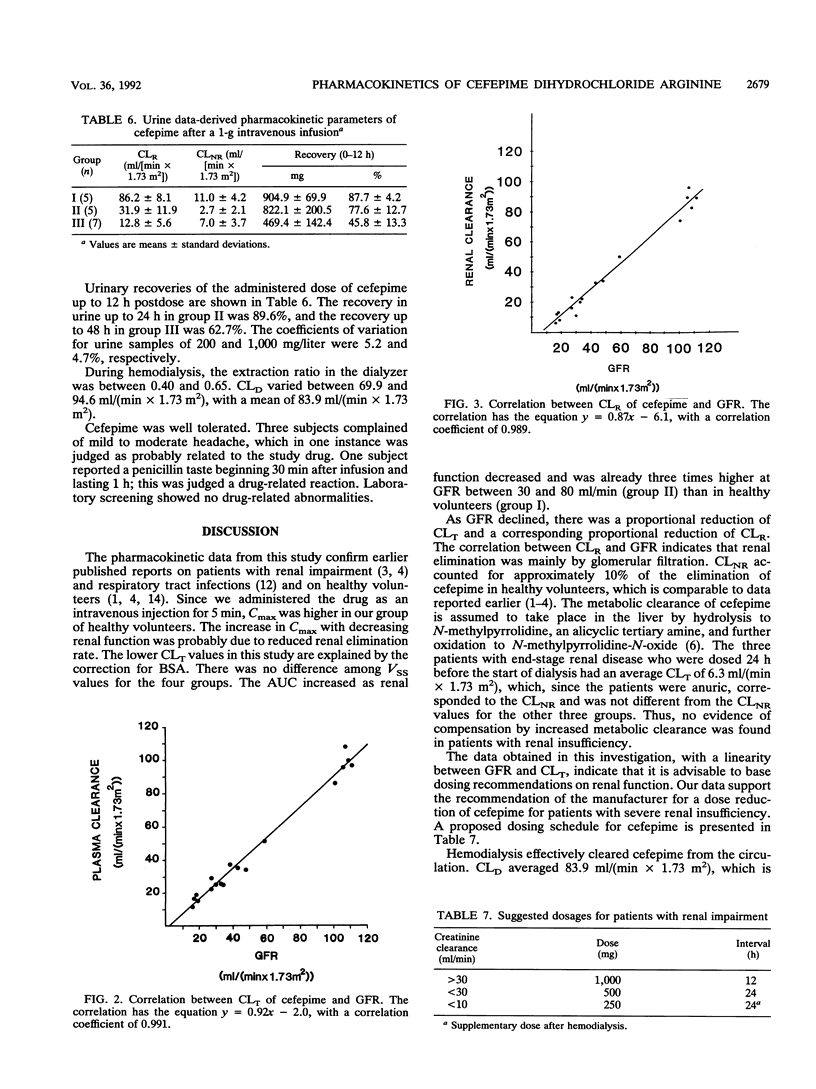
In this study, the safety, tolerance, and pharmacokinetics of a single 1-g intravenous dose of cefepime (BMY-28142) were investigated. Twenty-three volunteers with various degrees of renal function were assigned to four trial groups according to glomerular filtration rates (GFR). Group IV consisted of five patients with end-stage renal disease undergoing treatment with hemodialysis. Cefepime concentrations in samples from plasma, urine, and infusion solutions were assayed with high-pressure liquid chromatography. The volume of distribution corresponded to the assumed extracellular fluid volume and did not differ significantly between the four groups. The area under the concentration-time curve increased as renal function decreased; in group II (GFR, 31 to 80 ml/[min x 1.73 m2]; n = 6), it was already three times higher than in group I (GFR, > or = 80 ml/[min x 1.73 m2]; n = 5). Mean residence time was 2.4, 6.8, 11.4, and 31.6 h for the four groups, respectively. Total clearance decreased (97.2, 34.6, 19.8, and 6.3 ml/[min x 1.73 m2]) with decreasing renal function, and a linear relationship between total plasma clearance and GFR was found with the regression equation y = 0.92x-2.0 (r = 0.991). Renal clearance was linearly correlated to GFR with the regression equation y = 0.87x-6.1 (r = 0.989), indicating that renal elimination is mainly by glomerular filtration. During hemodialysis, the extraction ratios were between 0.40 and 0.65. Dialysis clearance varied between 69.9 and 94.6 ml/(min x 1.73 m2).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Forgue S. T., Gleason C. R., Knupp C. A., Pittman K. A., Weidler D. J., Martin R. R. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990 Jun;34(6):1118–1122. doi: 10.1128/aac.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Knupp C. A., Forgue S. T., Matzke G. R., Guay D. R., Pittman K. A. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990 Sep;48(3):268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- Barbhaiya R. H., Knupp C. A., Forgue S. T., Matzke G. R., Halstenson C. E., Opsahl J. A., Pittman K. A. Disposition of the cephalosporin cefepime in normal and renally impaired subjects. Drug Metab Dispos. 1991 Jan-Feb;19(1):68–73. [PubMed] [Google Scholar]
- Bodey G. P., Ho D. H., LeBlanc B. In vitro studies of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 Feb;27(2):265–269. doi: 10.1128/aac.27.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgue S. T., Kari P., Barbhaiya R. N-oxidation of N-methylpyrrolidine released in vivo from cefepime. Drug Metab Dispos. 1987 Nov-Dec;15(6):808–815. [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Barry A. L., Thornsberry C. Evaluation of the in vitro activity of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 May;27(5):679–682. doi: 10.1128/aac.27.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Bayer A. S. Efficacy of BMY-28142 in experimental bacteremia and meningitis caused by Escherichia coli and group B streptococci. Antimicrob Agents Chemother. 1985 Jul;28(1):51–54. doi: 10.1128/aac.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A., Boothman C., Phillips I. Comparative in vitro activity of cefpirome and cefepime, two new cephalosporins. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):677–685. doi: 10.1007/BF01964272. [DOI] [PubMed] [Google Scholar]
- Kovarik J. M., ter Maaten J. C., Rademaker C. M., Deenstra M., Hoepelman I. M., Hart H. C., Matzke G. R., Verhoef J. Pharmacokinetics of cefepime in patients with respiratory tract infections. Antimicrob Agents Chemother. 1990 Oct;34(10):1885–1888. doi: 10.1128/aac.34.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzén E., Bäck S. E., Nilsson-Ehle I., Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984 Dec;104(6):955–961. [PubMed] [Google Scholar]
- Nye K. J., Shi Y. G., Andrews J. M., Wise R. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother. 1989 Jul;24(1):23–28. doi: 10.1093/jac/24.1.23. [DOI] [PubMed] [Google Scholar]
- Phelps D. J., Carlton D. D., Farrell C. A., Kessler R. E. Affinity of cephalosporins for beta-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986 May;29(5):845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Maniatis A., Bertram M. A., Young L. S. In vitro activity of BMY-28142 in comparison with those of other beta-lactam antimicrobial agents. Antimicrob Agents Chemother. 1985 Apr;27(4):515–519. doi: 10.1128/aac.27.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täuber M. G., Hackbarth C. J., Scott K. G., Rusnak M. G., Sande M. A. New cephalosporins cefotaxime, cefpimizole, BMY 28142, and HR 810 in experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1985 Mar;27(3):340–342. doi: 10.1128/aac.27.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuye A., Pijck J. In vitro antibacterial activity of BMY-28142, a new extended-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 Apr;27(4):574–577. doi: 10.1128/aac.27.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]


