Abstract
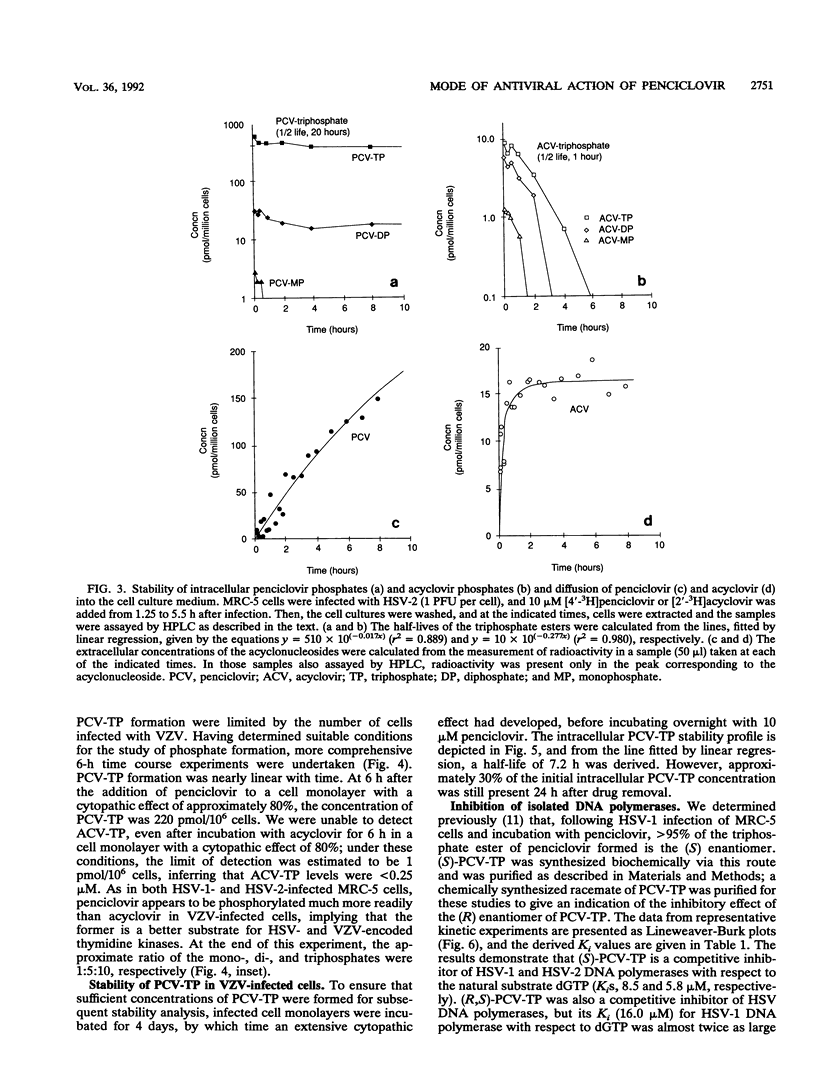
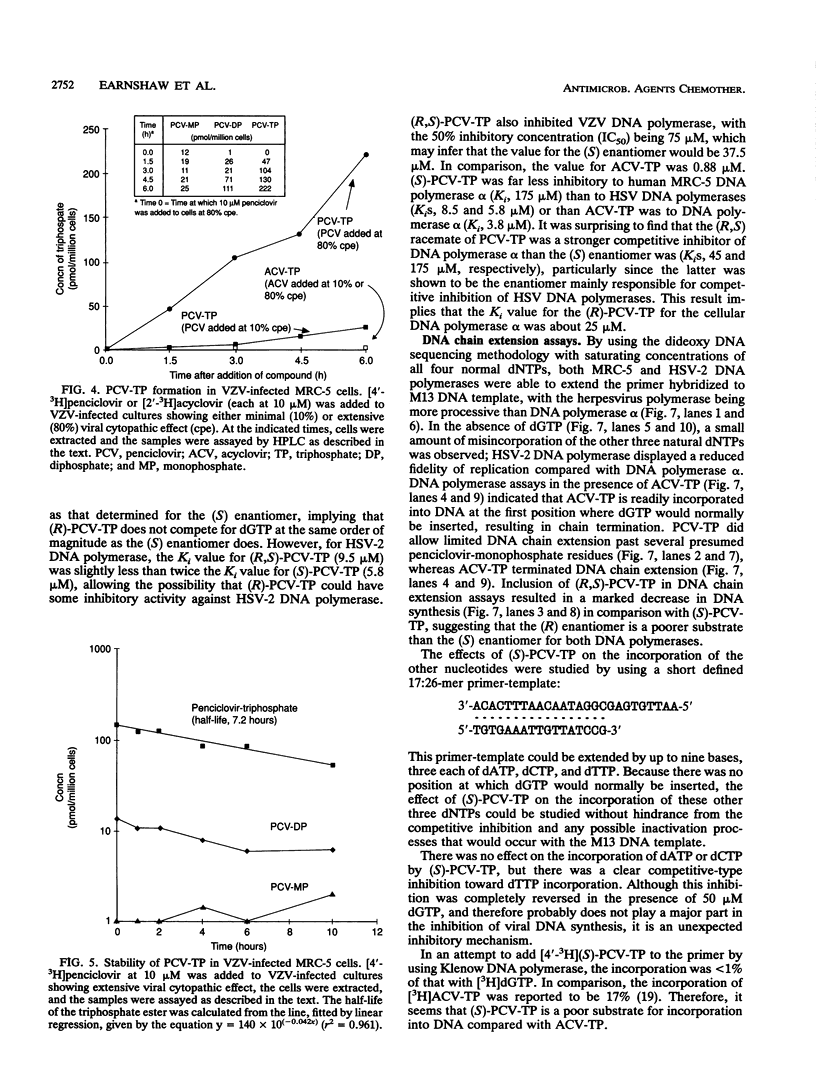
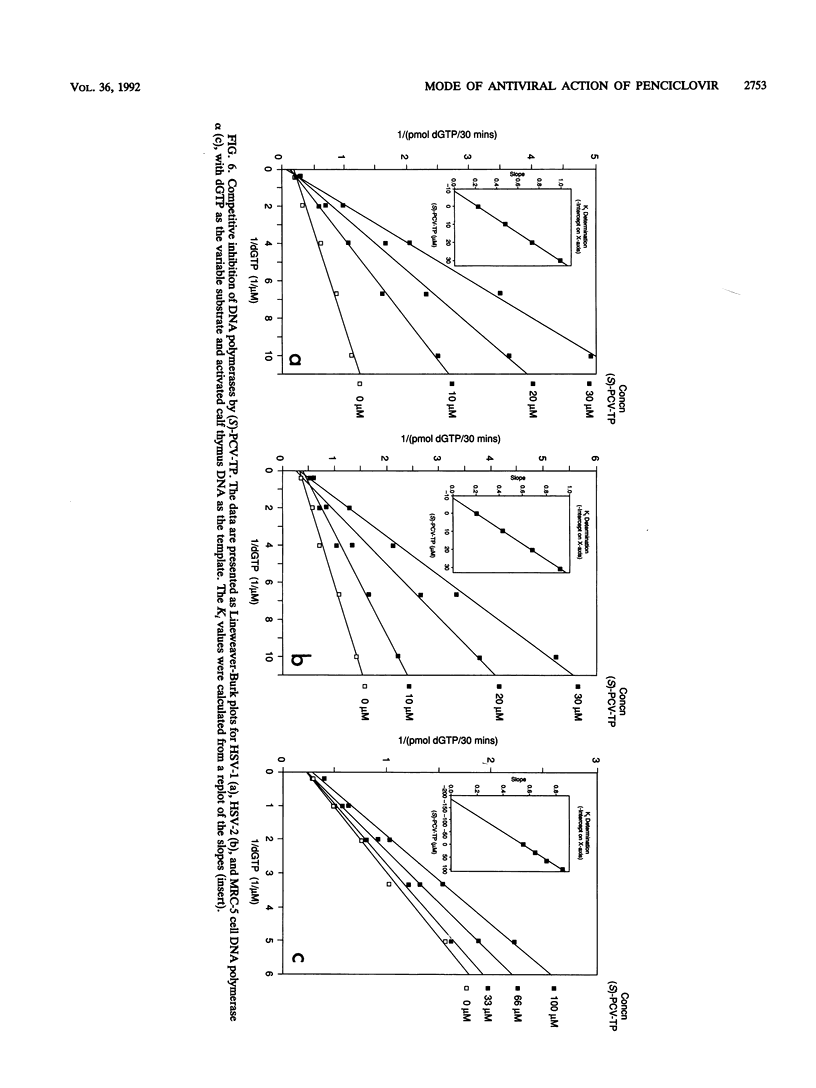
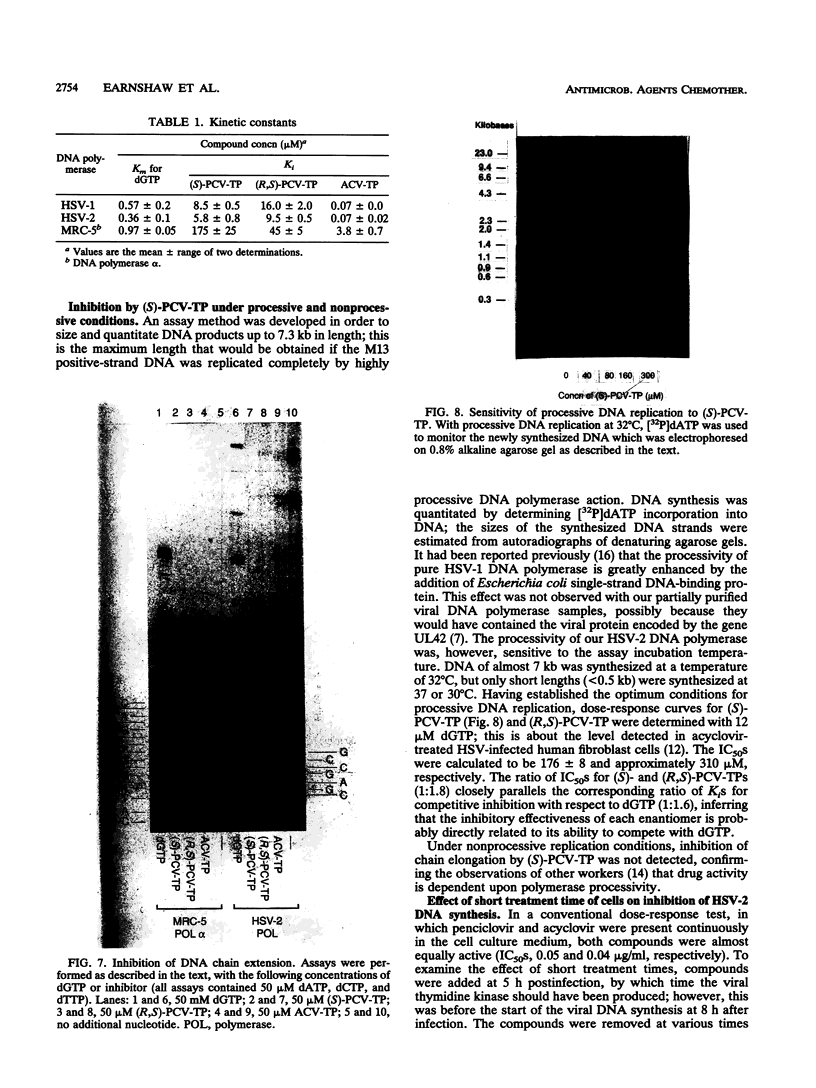
The metabolism and mode of action of penciclovir [9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine; BRL 39123] were studied and compared with those of acyclovir. In uninfected MRC-5 cells, low concentrations of the triphosphates of penciclovir and acyclovir were occasionally just detectable, the limit of detection being about 1 pmol/10(6) cells. In contrast, in cells infected with either herpes simplex virus type 2 (HSV-2) or varicella-zoster virus (VZV), penciclovir was phosphorylated quickly to give high concentrations of the triphosphate ester. Following the removal of penciclovir from the culture medium, penciclovir-triphosphate remained trapped within the cells for a long time (half-lives, 20 and 7 h in HSV-2- and VZV-infected cells, respectively). In HSV-2-infected cells, acyclovir was phosphorylated to a lesser extent and the half-life of the triphosphate ester was only 1 h. We were unable to detect any phosphates of acyclovir in VZV-infected cells. (S)-Penciclovir-triphosphate inhibited HSV-1 and HSV-2 DNA polymerase competitively with dGTP, the Ki values being 8.5 and 5.8 microM, respectively, whereas for acyclovir-triphosphate, the Ki value was 0.07 microM for the two enzymes. Both compounds had relatively low levels of activity against the cellular DNA polymerase alpha, with Ki values of 175 and 3.8 microM, respectively. (S)-Penciclovir-triphosphate did inhibit DNA synthesis by HSV-2 DNA polymerase with a defined template-primer, although it was not an obligate chain terminator like acyclovir-triphosphate. These results provide a biochemical rationale for the highly selective and effective inhibition of HSV-2 and VZV DNA synthesis by penciclovir and for the greater activity of penciclovir than that of acyclovir when HSV-2-infected cells were treated for a short time.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. R., Bacon T. H., Sutton D. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl) guanine (BRL 39123) in animals. Antimicrob Agents Chemother. 1988 Mar;32(3):358–363. doi: 10.1128/aac.32.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. R., Bacon T. H., Sutton D., Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987 Aug;31(8):1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles S. E., Pierce D. M. High-performance liquid chromatographic method for the determination of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL-39123) in human plasma and urine. Analyst. 1989 Nov;114(11):1373–1375. doi: 10.1039/an9891401373. [DOI] [PubMed] [Google Scholar]
- Gadler H., Larsson A., Sølver E. Nucleic acid hybridization, a method to determine effects of antiviral compounds on herpes simplex virus type 1 DNA synthesis. Antiviral Res. 1984 Apr;4(1-2):63–70. doi: 10.1016/0166-3542(84)90026-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb J., Marcy A. I., Coen D. M., Challberg M. D. The herpes simplex virus type 1 UL42 gene product: a subunit of DNA polymerase that functions to increase processivity. J Virol. 1990 Dec;64(12):5976–5987. doi: 10.1128/jvi.64.12.5976-5987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Hodge R. A., Perkins R. M. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob Agents Chemother. 1989 Feb;33(2):223–229. doi: 10.1128/aac.33.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson A. H., Harmenberg J. G., Wahren B. E. Influence of acyclovir and bucyclovir on nucleotide pools in cells infected with herpes simplex virus type 1. Antimicrob Agents Chemother. 1986 May;29(5):821–824. doi: 10.1128/aac.29.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick M. W., McGill J. I., White J. E., Wood M. J. Oral acyclovir in acute herpes zoster. Br Med J (Clin Res Ed) 1986 Dec 13;293(6561):1529–1532. doi: 10.1136/bmj.293.6561.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mul Y. M., van Miltenburg R. T., De Clercq E., van der Vliet P. C. Mechanism of inhibition of adenovirus DNA replication by the acyclic nucleoside triphosphate analogue (S)-HPMPApp: influence of the adenovirus DNA binding protein. Nucleic Acids Res. 1989 Nov 25;17(22):8917–8929. doi: 10.1093/nar/17.22.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasheuer H. P., Grosse F. Immunoaffinity-purified DNA polymerase alpha displays novel properties. Biochemistry. 1987 Dec 15;26(25):8458–8466. doi: 10.1021/bi00399a064. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E., Elias P., Lehman I. R. Processive replication of single-stranded DNA templates by the herpes simplex virus-induced DNA polymerase. J Biol Chem. 1987 Mar 25;262(9):4252–4259. [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. Nonstructural proteins of herpes simplex virus. I. Purification of the induced DNA polymerase. J Virol. 1977 Nov;24(2):618–626. doi: 10.1128/jvi.24.2.618-626.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon J. E. Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J Biol Chem. 1989 Nov 15;264(32):19039–19044. [PubMed] [Google Scholar]
- Reardon J. E., Spector T. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J Biol Chem. 1989 May 5;264(13):7405–7411. [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Vere Hodge R. A., Sutton D., Boyd M. R., Harnden M. R., Jarvest R. L. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-l-yl)guanine; penciclovir]. Antimicrob Agents Chemother. 1989 Oct;33(10):1765–1773. doi: 10.1128/aac.33.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Konno K., Mori S., Shigeta S., Kumagai M., Watanabe Y., Machida H. Mechanism of selective inhibition of varicella zoster virus replication by 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Mol Pharmacol. 1989 Aug;36(2):312–316. [PubMed] [Google Scholar]