Abstract
Myostatin, a member of the transforming growth factor-β superfamily, is a genetic determinant of skeletal muscle growth. Mice and cattle with inactivating mutations of myostatin have marked muscle hypertrophy. However, it is not known whether myostatin regulates skeletal muscle growth in adult men and whether increased myostatin expression contributes to wasting in chronic illness. We examined the hypothesis that myostatin expression correlates inversely with fat-free mass in humans and that increased expression of the myostatin gene is associated with weight loss in men with AIDS wasting syndrome. We therefore cloned the human myostatin gene and cDNA and examined the gene’s expression in the skeletal muscle and serum of healthy and HIV-infected men. The myostatin gene comprises three exons and two introns, maps to chromosomal region 2q33.2, has three putative transcription initiation sites, and is transcribed as a 3.1-kb mRNA species that encodes a 375-aa precursor protein. Myostatin is expressed uniquely in the human skeletal muscle as a 26-kDa mature glycoprotein (myostatin-immunoreactive protein) and secreted into the plasma. Myostatin immunoreactivity is detectable in human skeletal muscle in both type 1 and 2 fibers. The serum and intramuscular concentrations of myostatin-immunoreactive protein are increased in HIV-infected men with weight loss compared with healthy men and correlate inversely with fat-free mass index. These data support the hypothesis that myostatin is an attenuator of skeletal muscle growth in adult men and contributes to muscle wasting in HIV-infected men.
Muscle wasting is common in chronic illnesses, such as that associated with HIV and some types of cancer (1–5), and it produces debility, impairs quality of life, and portends poor disease outcome. The mechanism of muscle wasting in these diseases is not known.
Myostatin (growth differentiation factor 8, GDF-8), a member of the transforming growth factor-β superfamily, is a regulator of skeletal muscle growth (6, 7). Mice with null mutations of the myostatin gene have increased muscle mass (6). Similarly, mutations of the myostatin gene in cattle are associated with muscle hypertrophy (7–10). These data suggest that myostatin is a genetic determinant of skeletal muscle mass. However, we do not know whether myostatin expression is regulated in adult humans and whether its increased expression contributes to sarcopenia associated with HIV infection. We hypothesized that increased expression and secretion of myostatin may contribute to muscle wasting in HIV-infected men with weight loss. To test this hypothesis, we cloned the full-length human myostatin cDNA and examined the expression of myostatin mRNA and protein by Northern analysis, immunostaining, and Western blot analysis in muscle biopsy specimens from healthy and HIV-infected men. We developed an RIA to measure the serum myostatin concentrations in healthy men and HIV-infected men with weight loss and correlated these concentrations with fat-free mass index. These data support the hypothesis that myostatin is an important regulator of muscle growth in adult men and contributes to muscle wasting in HIV-infected men. The paper also describes the genomic organization and chromosomal mapping of the human myostatin gene.
MATERIALS AND METHODS
Generation of the Full-Length Human cDNA Sequence and Localization of Intron/Exon Junctions.
The expressed sequence tag (EST) database was screened with the mouse myostatin coding sequence (accession no. U84005; ref. 6), and partially overlapping homologous human sequences were arranged to generate a consensus 2.3-kb cDNA sequence. This sequence was confirmed by reverse transcription–PCR (11), using total RNA isolated from human vastus lateralis muscle tissue, and either a poly dT-20 mer primer or reverse (R) 20-mer deoxyribonucleotide primers spanning the whole consensus EST region. The 5′ ends of these antisense primers are located at the following positions (A at the initiation of translation being nucleotide 1): R13, 401; R15, 514; R16, 896; R21, 1274; R12, 1610; R14, 2200; R11, 2360; R10, 2422; and R17, 2645. Aliquots of human genomic DNA (0.5 μg) and the reverse-transcription reaction (1/10) were subjected to PCR with different combinations of reverse primers and the following forward (F) 20-mer primers for the myostatin-homologous human EST consensus sequence: F15, 392; F12, 515; F16, 879; F10, 1252; F11, 1818, and one for the 5′ end of the mouse gene (6) (P3: nucleotide 25, in this sequence). The 5′ cDNA sequence, not contained in the consensus EST, was obtained from the purified DNA fragments generated by PCR using a forward primer for the 5′ end of the mouse gene (6) (P3: nucleotide 25 in this sequence) paired with R13 and R15 on both RNA and DNA. PCR fragments for the region spanning from F12 through R21 in the DNA also were sequenced.
A human myostatin coding region cDNA construct was generated by inserting the F18/R23 cDNA fragment into the Srf site of the PCR-Script Amp Cloning vector (Stratagene) and selecting the sense clones by PCR with primers T3/F15 and T3/R13. This construct was digested with PstI and BsaI and ligated with the cDNA fragment liberated from EST 300367 with the same enzymes, thus generating the full-length myostatin cDNA.
The intron positions were determined by comparing homologous sequences from the cDNA and genomic DNA and confirmed by screening a human P1 library (Genome Systems) with the P3/R13 cDNA fragment labeled with 32P-dCTP. Two P-1 clones were detected and subjected to PCR with different primer pairs for the myostatin coding region. The fragments were sequenced to obtain the sequence of introns 1 and 2. In addition, the DNAs isolated from the yeast artificial chromosomes (YACs) containing the human myostatin gene (see below) were amplified by PCR and sequenced to verify the intron/exon junctions of intron 2.
Chromosomal Mapping.
A human monochromosome minimapping panel of somatic Chinese hamster ovary (CHO)/human cell hybrid DNAs (Coriell Cell Repositories, Camden, NJ) (12) was subjected to PCR with F10/R14 primers, encompassing a 0.95-kb band in the human DNA. A radiation-reduced somatic cell hybrid panel (Genebridge 4, Research Genetics, Huntsville, AL) (12) also was subjected to the same PCR amplification, and the results were analyzed by the Massachusetts Institute of Technology Netserver at the Whitehead Institute (http://carbon.wi.mit.edu:8000/cgi-bin/contig/rhmapper.pl). DNA was isolated from four YACs in this region and submitted to PCR to confirm the presence of the human myostatin gene.
Determination of Transcription Initiation Sites and mRNA Expression.
Total human skeletal muscle RNA was submitted to primer extension with 5′ 32P-labeled reverse primers located close to and downstream of the translation initiation codon at positions 54 (R19B) and 17 (R18) (13). The 5′ rapid amplification of cDNA ends (RACE) (14) was performed by using the Marathon-Ready cDNA 5′ RACE kit (CLONTECH) and human skeletal muscle poly(A)+ RNA preligated to a 5′ adaptor for anchoring a complementary AP1 adaptor primer. PCR was performed with AP1 and reverse primers used for primer extension.
Northern analysis was performed on poly(A)+ mRNA (2 μg/lane) from multiple human tissues (CLONTECH), by using a 2.3-kb, 32P-labeled cDNA probe from EST 300367. The blots were stripped and rehybridized with a human β actin cDNA.
Protein Expression by Western Blot.
We selected two 16-aa sequences within the deduced amino acid sequence of the protein encoded for by the human myostatin gene by using the macvector program and checked for their lack of homology to other proteins (7). The sequences started at residues 133 (peptide A) and 349 (peptide B) and are identical to the respective mouse myostatin sequences (6). Sequence B is common to myostatin and its precursor, and sequence A is present only in the myostatin precursor. The two peptides, GKPKCCFFKFSSKIQY and NMLYFNGKEQIIYGKI, respectively, were synthesized by the fluorenylmethoxycarbonyl solid-phase method and used to generate polyclonal antibodies in rabbits (Bethyl Laboratories, Montgomery, TX). The IgGs were purified by affinity chromatography, and the titer was determined by an enzyme-linked immunoassay.
For Western blot analysis, 20- to 50-mg sections of the mouse and human quadriceps muscle were homogenized in a buffer containing 20 mM Hepes (pH 7.2), 0.5 mM EDTA, 1 mM DTT, 10% glycerol, 3 μM leupeptine, 1 μM pepstatin A, 1 mM phenylmethylsulfonyl fluoride and fractionated (15) into a 15,000 g/60-min postmitochondrial supernatant fraction (S), and an extract of the particulate fraction with a buffer containing 50 mM Tris (pH 7.2), 0.1 mM EDTA, 0.1 mM EGTA, 12 mM 2-mercaptoethanol, 10% glycerol, and a mild nonionic detergent (20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate). The direct homogenate was obtained from 10–20 mg of tissue in a buffer containing 1% SDS, 1 mM vanadate, and 10 mM Tris hydrochloride, pH 7.4. Aliquots containing 15 μg of muscle protein or 0.2–3 μl of serum were heated at 95°C for 5 min in a denaturing/reducing buffer with 1% SDS and 1.25% 2-mercaptoethanol, and analyzed on 12% polyacrylamide gels. The proteins were electroblotted (11, 15) and detected with antimyostatin antibody B (1:1,000), immunoperoxidase-linked anti-rabbbit IgG (1:2,000), and a luminol-based reaction on x-ray film. In certain cases the nitrocellulose membranes were stripped of bound antibodies and reacted with antimyostatin precursor antibody A. Band density was measured by densitometry. To verify specificity, nonimmune rabbit IgG was applied to sections of the membrane blots instead of antipeptide antibody. Other blots were incubated with antibody in the presence of a large molar excess of the corresponding peptide. In certain experiments, 2-mercaptoethanol and SDS concentrations were 5% and 1%, respectively, in both the sample and running buffers.
Immunohistochemical Staining of Tissue Sections.
Four-micrometer paraffin sections of formalin-fixed human skeletal muscle and other tissues were deparaffinized, hydrated, and pretreated for antigen retrieval in 10 mM citrate buffer, pH 6.0 in a microwave pressure cooker for 15 min. Endogenous peroxidase activity was blocked with 3% H2O2 for 15 min, and nonspecific binding was blocked with 5% protein blocker (Signet Laboratories, Dedham, MA) for 10 min. Sections were incubated in primary antibodies (1:500) overnight at 22–24°C, with biotinylated linking reagent (Signet; 1:3) for 45 min, followed by diaminobenzidine for 5 min at 22–24°C. Sections were counterstained with hematoxylin. Serial sections were stained with a mAb against skeletal myosin (fast), which preferentially stains type 2 fibers (16).
RIA.
Myostatin peptide B was iodinated by using the iodogen method and incubated (25,000 cpm) with 50 μl of serum and 2.9 μg of antipeptide B IgG in 500 μl of PBS at pH 7.4, 4°C, for 24 hr to achieve 25–30% specific binding. Free and IgG-bound peptide were separated with 6% polyethylene glycol and secondary anti-rabbit IgG antibody (17). A four-parameter logistics program was used to calculate the concentration of the unknown from the reference displacement curve generated by using graded concentrations of peptide B.
Human Subjects and Body Composition Analysis.
The institutional review boards of the Harbor-University of California-Los Angeles Research and Education Institute, Torrance, CA, and Washington University School of Medicine, St. Louis, MO, approved the protocols. All participants signed a written, informed consent. Fat-free mass of healthy men was determined by underwater weighing (18). In HIV-infected men, body composition was determined by dual-energy x-ray absorptiometry by Hologic 4500 scanner (19).
Statistical Analyses.
Because serum concentrations of myostatin-related protein were not normally distributed and did not meet the assumptions of ANOVA, the data were log-transformed. Serum concentrations of myostatin-related protein in the three groups were compared by using one-way ANOVA. If ANOVA demonstrated significant overall differences, then between-group comparisons were made by using the t tests. Correlations between serum myostatin-related protein concentrations and fat-free mass, fat-free mass index, and body mass index were performed by the Pearson Product Moment Correlation. Data are reported as mean + SEM. P values of less than 0.05 were considered significant.
RESULTS
The Human Myostatin Gene Contains Two Introns.
We screened the human EST data bank for sequences homologous to the mouse myostatin (6) and generated from overlapping EST fragments a consensus cDNA sequence that extended from the 3′ poly(A)+ end to a position homologous to nucleotide 481 on the mouse myostatin sequence. To characterize the remaining 5′ region not contained in the human EST fragments (Fig. 1), total human skeletal muscle RNA was submitted to reverse transcription–PCR with either the reverse poly dT primer or the different reverse myostatin primers. The full-length myostatin cDNA was cloned by ligating the longest human EST available to the partially overlapping cDNA product generated by reverse transcription of human skeletal muscle RNA (Fig. 1). The resulting cDNA construct contained the complete myostatin coding region from nucleotides −3 to 2001, with a stop codon at nucleotide 1084 (Fig. 1).
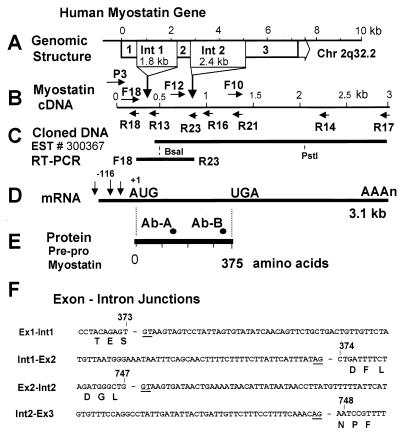
Figure 1.
Human myostatin gene and its products. (A) Position and size of introns 1 and 2. (B) Myostatin cDNA indicating the positions of the intron/exon junctions (vertical arrows) and forward (F) and reverse (R) human primers; P3 is the only mouse primer. (C) Cloning strategy indicating Pflm1 and PstI sites. (D) Human skeletal muscle mRNA with its transcriptional initiation sites. (E) Myostatin protein precursor with positions of synthetic peptides used to raise the antibodies. (F) Sequence of exon/intron junctions limited to the first 60 bases in the intron and the 10 contiguous bases in the exon.
The sizes of the PCR fragments generated from human genomic DNA by using all combinations of primers downstream of F-16 (complementary to R-16) coincided with those generated from the cDNA, indicating the absence of introns in this region. However, two PCR fragments much larger than expected from the cDNA sequence were generated when we used primers P3/R13 and F12/R16, suggesting the presence of two introns. We confirmed the sequences of introns 1 and 2 and their junctions with the flanking exons (Fig. 1) by subcloning them from P1 genomic clones that contained the human myostatin gene. The 1,789-bp intron 1 is located between nucleotides 372 and 373 of the cDNA sequence. Intron 2 is 2.4 kb long and follows nucleotide 747. The three exons encode 125, 124, and 126 amino acids, respectively.
The Human Myostatin Gene Maps to Chromosome 2q.32.2 and Is Represented on Several YACs.
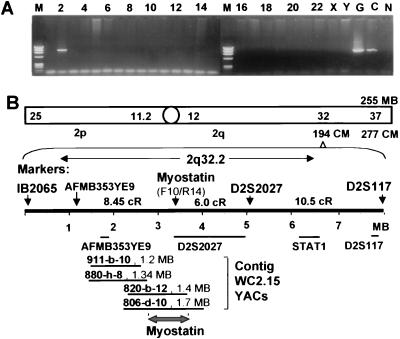
We used a somatic cell hybrid panel to map the myostatin gene to human chromosome 2 (Fig. 2A). The radiation hybrid panel localized the human myostatin gene to chromosomal region 2q32.2, 8.45 cR distal to marker AFMB35YE9, and 6.0 cR proximal to the WI3652 (D2S2027) marker with a logarithm of odds score >17 (Fig. 2B). PCR screening of YAC clones within contig WC2.15 revealed that YACs 820-b-12 and 806-d-10 contained the myostatin gene. These parameters helped to more precisely assign the human myostatin gene to the distal part of YAC 820-b-12 within contig WC2.15 of chromosome region 2q32.2.
Figure 2.
Chromosomal mapping of the human myostatin gene. (A) DNA fragments amplified from the human somatic cell hybrid panel by using primers F10/R14. Chromosomes are indicated at the top of each lane. G, human genomic DNA; C; human myostatin cDNA; N, hamster control. (B) Schematic representation of genetic markers and the YACs corresponding to chromosome region where myostatin was mapped by the radiation-reduced somatic cell hybrid panel. Marker D2S2027 also is named WI3652. The framework markers at 144 cM are shown; map distances are given in centiRays (cR) or in the lower YAC map as MB (conversion factor, 3.7 cR/MB).
Myostatin RNA Is Selectively Expressed in Human Skeletal Muscle from Several Transcription Initiation Sites.
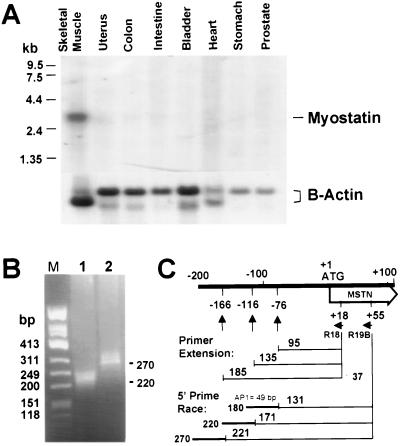
We hybridized a Northern blot containing poly(A)+ RNA isolated from several human organs with myostatin and β-actin cDNA probes. A single 3.1-kb mRNA species was seen in the human skeletal muscle, but not in uterus, colon, small intestine, bladder, heart, stomach, or prostate (Fig. 3A).
Figure 3.
Expression of myostatin mRNA in the human skeletal muscle. (A, Upper) Northern blot with poly(A)+ RNA hybridized with a P3/R13 myostatin cDNA probe. (A, Lower) The same blot hybridized with β-actin cDNA probe. (B) 5′ rapid amplification of cDNA ends/PCR-amplified products obtained with primers R19B/AP1. The use of vector primer AP1 results in a cDNA 49 nt longer than the original reverse-transcribed RNA. (C) Schematic representation of the 5′ untranslated region of the human skeletal muscle myostatin mRNA.
We established the mRNA start sites by performing primer extension analysis using primer R18 on total RNA isolated from skeletal muscle (Fig. 1). Three bands were identified corresponding to putative transcription initiation sites at nucleotides −76, −116, and −166 (Fig. 3C). The identity of the transcripts was confirmed by 5′ rapid amplification of cloned ends of a human skeletal muscle cDNA library. The sizes of the amplified cDNAs are consistent with the expected size of the transcripts that have initiation at nucleotides −166 and −116 (Fig. 3B).
A Myostatin-Related Protein Is Expressed in Human Skeletal Muscle and Secreted into Plasma.
To characterize myostatin protein in the skeletal muscle and serum, we generated two polyclonal, affinity-purified antibodies against synthetic peptides whose relative position is shown in Fig. 1. Antibody A was designed to recognize the precursor protein, and antibody B both the precursor and the mature myostatin protein, as predicted from the posttranslational processing described in CHO cells (6).
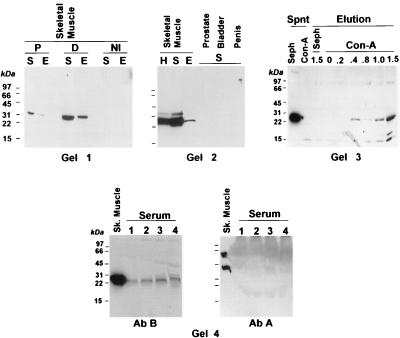
We used these antibodies in Western blots to examine the expression of myostatin and its precursor in mouse skeletal muscle and several human tissues and its presence in serum. A single 26-kDa band is apparent in the supernatant, and the membrane extract of mouse skeletal muscle on the blot reacted with antibody B (Fig. 4, Gel 1). The absence of larger immunoreactive proteins indicates that little or no myostatin precursor exists in the muscle extracts. Competition with peptide B reduced the intensity of the 26-kDa band, and nonimmune IgG did not produce this band, providing evidence of specificity. The 26-kDa protein detected with antibody B was not observed when the blot was incubated with antibody A (not shown), suggesting that little or no myostatin precursor is present in the skeletal muscle. A higher molecular mass band of lesser intensity than the 26-kDa band was detected occasionally in some human muscle preparations; the nature of this band is not known.
Figure 4.
Western blot of myostatin-related protein in the skeletal muscle and human serum. Tissue extracts were prepared from mouse (Gel 1) and human skeletal muscle (Gel 2) and fractionated into supernatant (S) and membrane extracts (E). H, unfractionated homogenate. (Gel 1) The Western blot membranes were cut into three sections and reacted with either nonimmune IgG (NI) or antibody B directly (D), or in the presence of an excess of the corresponding peptide (P). (Gel 2) The S fraction also was prepared from human prostate, bladder, or penile corpora cavernosa. (Gel 3) The S fraction from the human skeletal muscle was incubated with either Con A-Sepharose (Con-A) or Sepharose (Seph). After centrifugation, both the supernatant (Spnt) and the eluates with increasing concentrations of α-methyl-d-mannoside (Elution) were submitted to Western blot assays with antibody B. (Gel 4, Left) The S fraction of the human skeletal muscle and increasing amounts of human serum (lane 1, 0.2 μl; lane 2, 0.5 μl; lane 3, 1 μl, lane 4, 2 μl) were subjected to Western blot assays with antibody B. (Gel 4, Right) The blot from Gel 4, Left was stripped and reacted with antibody A.
Apart from the species difference, the 26-kDa protein detected with antibody B in mouse skeletal muscle fractions also was seen in the human skeletal muscle homogenate, the supernatant, and the membrane fractions (Fig. 4, Gel 2). The 26-kDa band was absent in tissues rich in smooth muscle, such as the prostate, urinary bladder, and penile corpora cavernosa.
The larger size of the band detected with antibody B in the human skeletal muscle, as compared with the protein detected in CHO cells, transfected with the mouse myostatin cDNA (6) appears to be caused, at least in part, by glycosylation. This protein modification was shown by incubating the human skeletal muscle supernatant with Con A-linked Sepharose, which binds some types of glycoproteins (20). The 26-kDa band persists in the supernatant after incubation of the muscle extract with control Sephadex beads, but is eliminated from supernatant after its incubation with Con A-Sepharose (Fig. 4, Gel 3). This band is eluted with high concentrations of α-methyl d-mannoside.
The 26-kDa protein recognized by antibody B in the human skeletal muscle is also present in human serum (Fig. 4, Gel 4, Left); the intensity of this band increases as serum volume is increased. No immunoreactive band was seen in the serum when the blot was subsequently reacted with antibody A (Fig. 4, Gel 4, Right), indicating that myostatin precursor is not present in circulation in detectable amounts.
Because the size of the myostatin-immunoreactive protein detected in muscle and serum by antibody B is about double the size of the 106-aa monomer expressed in heterologous CHO cells (6), we assessed whether the 26-kDa band is a dimer. Accordingly, we performed PAGE under more extreme denaturing and reducing conditions. However, no change in migration was noticed (not shown). Because of this discrepancy between the size of the immunoreactive band detected in human serum and muscle and that reported for mouse myostatin in CHO cells, we have designated the 26-kDa protein as a myostatin-immunoreactive protein until its identity is established.
Myostatin Immunostaining.
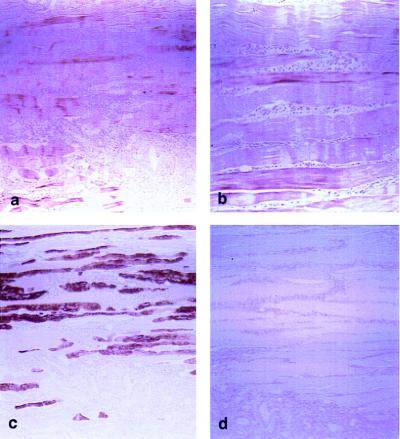
We observed myostatin immunoreactivity in human skeletal muscle fibers (Fig. 5 a and b), but not in the surrounding fibroconnective tissue or in small and large intestine, urinary bladder, prostate, or kidney. Myostatin immunostaining was present in equal intensity in both type 2 fibers (Fig. 5c) and type I fibers not stained by the antimyosin antibody (compare Fig. 5c with Fig. 5a). Preabsorption of primary antiserum with peptide B abolished the staining (Fig. 5d). No staining was observed with preimmune serum.
Figure 5.
Immunohistochemical staining. (a) Sections of skeletal muscle were incubated with antibody B (×50). (b) Higher magnification (×100) of a. (c) Serial sections were stained with myosin antibody to identify type I and II fibers. (d) Negative control with antibody B preadsorbed with 7 μg/liter of peptide B.
Increased Concentrations of Myostatin-Immunoreactive Protein in the Serum and Skeletal Muscle of HIV-Infected Men.
We developed an RIA to measure the serum concentrations of myostatin-related protein by using 125I-labeled peptide B as the tracer and antibody B. The sensitivity of the assay was 10.5 ng of peptide-equivalent/ml. Intra-assay coefficient of variation was 12.6% at the 20% point, 10.3% at the 50% point, and 11.7% at the 80% point. Inter-assay coefficient of variation was 8.8%. The cross-reactivity of related proteins inhibin B, activin A, and transforming growth factor-β1 in the myostatin RIA was less than 1.0%. Serial dilutions of human serum (1:1, 1:2, and 1:5) produced predictable decreases in the measured immunoreactivity and yielded a displacement curve that was parallel to that produced by graded concentrations of peptide B.
We measured serum myostatin concentrations in 42 healthy men, 19–65 years of age, free of any active disease, and between 95–115% of their ideal body weight (Table 1). We also studied two groups of HIV-infected men who were not acutely ill and not taking anabolic agents. Group I consisted of 38 HIV-infected men who either had not lost weight or lost less than 10% body weight in the preceding 6 months. Group II consisted of 23 HIV-infected men who had lost at least 10% of their body weight in the preceding 6 months and therefore had met the definition of AIDS wasting syndrome.
Table 1.
Baseline characteristics of the participants
| Characteristic | Healthy men, n = 42 | HIV-infected men with 0–10% weight loss, n = 38 | HIV-infected men with >10% weight loss, n = 23 |
|---|---|---|---|
| Age, years | 31.2 ± 1.9 | 41.7 ± 1.4 | 38.5 ± 1.4 |
| Weight, kg | 79.3 ± 1.6 | 74.1 ± 1.4 | 63.0 ± 2.5 |
| Height, m | 1.79 ± 0.01 | 1.74 ± 0.01 | 1.79 ± 0.02 |
| BMI, kg/m2 | 24.9 ± 0.5 | 23.8 ± 0.7 | 20.1 ± 0.8 |
| FFM, kg | 67.2 ± 1.3 | 53.4 ± 1.5 | 49.9 ± 2.2 |
| FFM index, kg/m2 | 21.0 ± 0.4 | 17.6 ± 0.4 | 15.5 ± 0.6 |
| CD4+ T cells/cmm | Not measured | 224 ± 37 | 199 ± 49 |
| CD8+ T cells/cmm | Not measured | 769 ± 75 | 367 ± 127 |
| Plasma HIV RNA copy number/ml | Not measured | 23,421 ± 11,333 | 172,613 ± 104,374 |
Data are mean ± SEM. BMI, body mass index; FFM, fat-free mass.
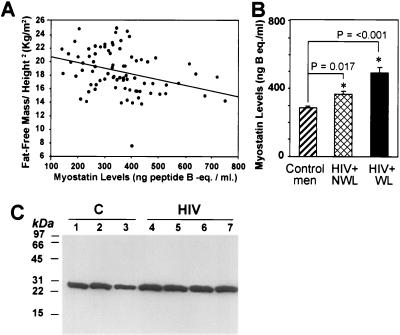
We established the reference range for serum myostatin concentrations in healthy, young men (mean ± SE: 290.5 ± 12.9 ng of peptide B equivalent/ml; range: 140–490 ng of peptide B equivalent/ml; n = 42). Serum concentrations of myostatin-immunoreactive protein were inversely correlated with fat-free mass (r = −0.30, P = 0.007) in healthy and HIV-infected men with different degrees of weight loss (Fig. 6A). Serum myostatin immunoreactivity also correlated inversely with fat-free mass index (fat-free mass divided by height2), r = −0.33, P = 0.003 (Fig. 6A), and body mass index, r = −0.30, P = 0.02. In HIV-infected men, serum myostatin-related protein concentrations were not significantly correlated with CD4+ and CD8+ T cell counts or plasma HIV-RNA copy number.
Figure 6.
Serum and intramuscular concentrations of myostatin-related protein in healthy and HIV-infected men. (A) Correlation of serum myostatin immunoreactivity and fat-free mass index (fat free mass/height2) in healthy and HIV-infected men. r = 0.33; P < 0.003. (B) Serum myostatin-related protein levels in healthy and HIV-infected men. The HIV-infected men without wasting (HIV+ NWL) either had not lost weight or had lost less than 10% body weight in the preceding 6 months. The HIV-infected men with wasting (HIV+ WL) had lost more than 10% of their premorbid weight in the preceding 6 months. (C) Western blot of the 26-kDa myostatin-related protein in skeletal muscle biopsy from healthy (C, lanes 1–3) and HIV-infected men (HIV, lanes 4–7).
Serum concentrations of myostatin-related protein were significantly different in healthy and the two groups of HIV-infected men, F (F statistic) = 20.7, DF (degrees of freedom) = 2, P < 0.001. Serum myostatin-immunoreactive protein concentrations were higher in HIV-infected men without wasting than healthy men (363.3 ± 21.1 vs. 290.5 ± 12.9 ng of peptide B equivalent/ml, P < 0.001) (Fig. 6B). HIV-infected men with weight loss in excess of 10% of premorbid weight had higher serum concentrations than HIV-infected men who either had not lost weight or lost less than 10% body weight (499.7+37.1 vs. 363.3+21.1 ng of peptide B equivalent/ml, P = < 0.001).
Western blots of muscle homogenates from HIV-infected men with >10% weight loss (Fig. 6C) revealed that the intensity of the 26-kDa band was increased in comparison to healthy men.
DISCUSSION
We have described the genomic organization and chromosome mapping of the human myostatin gene, the position and size of the two introns, and the sequence of the intron/exon junctions in the 7.7-kb region of the gene that is transcribed in the skeletal muscle. We have determined its precise location on chromosome 2q32.2, elucidated the transcriptional initiation sites, and cloned its full-length cDNA. Myostatin-immunoreactive protein is expressed uniquely in the human skeletal muscle as a 26-kDa protein and is secreted into plasma. The data from the Con A-Sepharose chromatography suggests that myostatin is glycosylated. We used an RIA to demonstrate that serum concentrations of myostatin-immunoreactive protein correlate inversely with fat-free mass and are increased in HIV-infected men with weight loss. These data support the proposed role of myostatin as an inhibitor of muscle growth in healthy and HIV-infected men.
The third exon of human myostatin gene codes for a sequence present in the putative mature protein. No introns are present in the 5′ untranslated region upstream of the initiation codon. This general scheme agrees with the genomic organization of other members of the transforming growth factor-β family (21). Intron 1 does not contain any cryptic exon/intron junction; however, intron 2 has not been completely sequenced, and we cannot exclude the possibility of alternative splicing sites in this region.
We have refined the localization of the human myostatin gene within the chromosomal region 2q32.2 and constructed a more detailed position map than the one previously reported (7) by identifying two overlapping YACs that contain the myostatin gene. Nebulin, another skeletal muscle gene, is contained in these YACs (22); however, other clusters of muscle genes have been mapped to other chromosomes (23, 24).
Northern and Western blots of human tissues and immunohistochemical staining demonstrate exclusive expression of myostatin mRNA and protein in the human skeletal muscle consistent with previous mouse data (6).
The in vivo processing of the human myostatin protein may be different from that of its mouse ortholog in an in vitro heterologous system, but similar to that of mouse myostatin in vivo. We have provided several lines of evidence to establish the specificity of antibody B for the myostatin mature protein, which suggests that the muscle and serum myostatin immunoreactivity is caused primarily by the 26-kDa myostatin-immunoreactive protein. The antibody detects a single 26-kDa band in the human and mouse skeletal muscle whose intensity is diminished by competition with excess of synthetic peptide B. Incubation of Western blots with preimmune rabbit Ig did not reveal this band. The 26-kDa band visualized on Western blots of human serum was identical in size to that observed in the human and mouse skeletal muscle. Finally, the 26-kDa myostatin-immunoreactive band was not observed in the smooth muscle of the prostate, bladder, or corpora cavernosa, consistent with the unique expression of myostatin mRNA in the skeletal muscle.
In CHO cells, murine myostatin was expressed as 102- and 30-kDa proteins under nonreducing conditions (6). Under reducing conditions, the predominant forms were 51 and 15 kDa, respectively, leading to the proposition that myostatin was present in that in vitro system in a dimeric form. The discrepancy in the apparent molecular masses between the previous report and our experiments appears to be caused by glycosylation of the 26-kDa band in the human muscle as opposed to CHO cells, as is the case for transforming growth factor-β receptors, activin, inhibin, and luteinizing hormone (20). The posttranslational processing of the human myostatin in the muscle in vivo may be different from that of murine myostatin in the transformed cell line used in the previous in vitro studies. Finally, myostatin may exist in the form of dimers that are not cleaved under the conditions used in our experiments. We have referred to the immunoreactive form of myostatin found in human serum and muscle as the myostatin-immunoreactive protein to distinguish it from the murine protein previously described in CHO cells. The immunostaining of the human skeletal muscle fibers by antibody B, and the lack of immunoreactivity in other tissues, agrees with our interpretation that the myostatin-immunoreactive protein is the processed form of myostatin in human muscle.
We provide evidence that a 26-kDa myostatin-immunoreactive protein circulates in the serum; the myostatin precursor protein was not detectable in serum. It is possible that this protein may have endocrine effects.
Because pure myostatin protein is not yet available, we used synthetic peptide B as the reference standard. Therefore, the reported serum concentrations reflect relative levels and not absolute concentrations. The serum concentrations of myostatin-immunoreactive protein are higher in HIV-infected men than in healthy men; these levels are even higher in HIV-infected men who meet the definition of AIDS wasting syndrome. There is an inverse correlation between myostatin-immunoreactive protein concentrations and fat-free mass index. These data support the hypothesis that increased intramuscular expression and secretion of myostatin or myostatin-immunoreactive protein contribute to the multifactorial pathophysiology of muscle protein wasting in HIV-infected men.
The mechanisms by which myostatin may contribute to muscle wasting are not known. We do not know whether myostatin acts directly on the skeletal muscle or centrally on the regulatory centers that determine body composition. A balance between muscle protein synthesis and breakdown and regenerative mechanisms maintains the muscle protein mass. It is not known whether myostatin induces apoptosis (25) or impairs tissue repair by inhibiting cell replication or muscle cell fiber growth (26, 27), or whether it has more generalized effects on protein and energy metabolism (28). The presence of significant circulating levels of myostatin-immunoreactive protein suggests that receptors for this protein might exist in the muscle and other sites that are involved in the metabolic regulation of body composition.
The higher levels of myostatin-immunoreactive protein in HIV-infected men with wasting and the development of muscle hypertrophy in myostatin-null mice raise the possibility that inhibition of myostatin expression, or of its binding to a putative receptor, might have therapeutic applications in muscle protein wasting associated with HIV infection, cancer cachexia, and old age.
Acknowledgments
This study was supported by National Institutes of Health Grants 1RO1DK49296, 1RO1AG13297, and 1RO1DK49393; Food and Drug Administration Grant OPD 1397; and Research Center Minority Institution (RCMI) Grants P20RR11145-01 (RCMI Clinical Research Initiative) and G12RR03026.
ABBREVIATIONS
- GDF-8
growth differentiation factor 8
- EST
expressed sequence tag
- YAC
yeast artificial chromosome
- CHO
Chinese hamster ovary
- F
forward
- R
reverse
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF104922).
References
- 1.Sellmeyer D E, Grunfeld C. Endocr Rev. 1996;17:518–532. doi: 10.1210/edrv-17-5-518. [DOI] [PubMed] [Google Scholar]
- 2.Hellerstein M K, Kahn J, Mundie H, Viteri F. Semin Oncol. 1990;17:17–23. [PubMed] [Google Scholar]
- 3.Kotler D P, Tierney A R, Wang J, Pierson R N., Jr Am J Clin Nutr. 1989;50:444–447. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- 4.Mulligan K, Tai V W, Schambelan M. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;15:43–48. doi: 10.1097/00042560-199705010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bhasin S, Tenover J S. J Clin Endocrinol Metab. 1997;82:1659–1660. doi: 10.1210/jcem.82.6.4061. [DOI] [PubMed] [Google Scholar]
- 6.McPherron A C, Lawler A M, Lee S-J. Nature (London) 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 7.McPherron A C, Lee S-J. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambadur R, Sharma M, Smith T P, Bass J J. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 9.Grobet L, Martin L J, Poncelot D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, et al. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 10.Charlier C, Coppieters W, Farnir F, Grobet L, Leroy P L, Michaux C, Mni M, Schwers A, Vanmanshoven P, Hanset R, et al. Mamm Genome. 1995;6:788–792. doi: 10.1007/BF00539005. [DOI] [PubMed] [Google Scholar]
- 11.Garban H, Marquez D, Magee T, Moody J, Rajavashisth T, Rodriguez J A, Hung A, Vernet D, Rajfer J, Gonzalez-Cadavid N F. Biol Reprod. 1997;56:954–963. doi: 10.1095/biolreprod56.4.954. [DOI] [PubMed] [Google Scholar]
- 12.Kastury K, Taylor W E, Gutierrez M, Ramirez L, Coucke P J, Van Hauwe P, Van Camp G, Bhasin S. Genomics. 1997;44:362–364. doi: 10.1006/geno.1997.4903. [DOI] [PubMed] [Google Scholar]
- 13.Taylor W E, Najmabadi H, Strathearn M, Jou N T, Liebling M, Rajavashisth T, Channani N, Pfung L, Bhasin S. Endocrinology. 1996;137:5407–5414. doi: 10.1210/endo.137.12.8940364. [DOI] [PubMed] [Google Scholar]
- 14.Frohman M A. In: PCR Protocols: A Guide to Methods and Applications. Innis M A, Gelfand J J, Sninsky J J, White T J, editors. San Diego: Academic; 1990. pp. 28–38. [Google Scholar]
- 15.Penson D F, Ng C, Rajfer J, Gonzalez-Cadavid N F. Endocrinology. 1997;138:3925–3932. doi: 10.1210/endo.138.9.5402. [DOI] [PubMed] [Google Scholar]
- 16.Jay V, Bekcer L E. Arch Pathol Lab Med. 1994;118:917–918. [PubMed] [Google Scholar]
- 17.Sinha-Hikim I, Arvar S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson J C, Landgren B M, Mazer N A, Bhasin S. J Clin Endocrinol Metab. 1998;83:1312–1318. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- 18.Bhasin S, Storer T W, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell T J, Tricker R, Shirazi A, Casaburi R. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 19.Bhasin S, Storer T W, Asbel-Sethi N, Kilbourne A, Hays R, Sinha-Hikim I, Shen R, Arver S, Beall G. J Clin Endocr Metabol. 1998;83:3155–3162. doi: 10.1210/jcem.83.9.5079. [DOI] [PubMed] [Google Scholar]
- 20.Mason A J, Farnworth P G, Sullivan J. Mol Endocrinol. 1996;10:1055–1065. doi: 10.1210/mend.10.9.8885240. [DOI] [PubMed] [Google Scholar]
- 21.Guron C, Sudarshan C, Raghow R. Gene. 1995;165:325–326. doi: 10.1016/0378-1119(95)00460-n. [DOI] [PubMed] [Google Scholar]
- 22.Limongi M Z, Pelliccia F, Rocchi A. Cytogenet Cell Genet. 1997;77:259–260. doi: 10.1159/000134590. [DOI] [PubMed] [Google Scholar]
- 23.Pallavicini A, Zimbello R, Tiso N, Muraro T, Rampoldi L, Bortoluzzi S, Valle G, Lanfranchi G, Danieli G A. Hum Mol Genet. 1997;6:1445–1450. doi: 10.1093/hmg/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 24.Tiso N, Rampoldi L, Pallavicini A, Zimbello R, Pandolfo D, Valle G, Lanfranchi G, Danieli G A. Biochem Biophys Res Commun. 1997;230:347–350. doi: 10.1006/bbrc.1996.5958. [DOI] [PubMed] [Google Scholar]
- 25.Allen D L. Am J Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- 26.Kelley G. J Appl Physiol. 1996;81:1584–1588. doi: 10.1152/jappl.1996.81.4.1584. [DOI] [PubMed] [Google Scholar]
- 27.Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Cytokine Growth Factors Rev. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- 28.Macallan D C. N Engl J Med. 1995;333:83–88. doi: 10.1056/NEJM199507133330202. [DOI] [PubMed] [Google Scholar]