Abstract
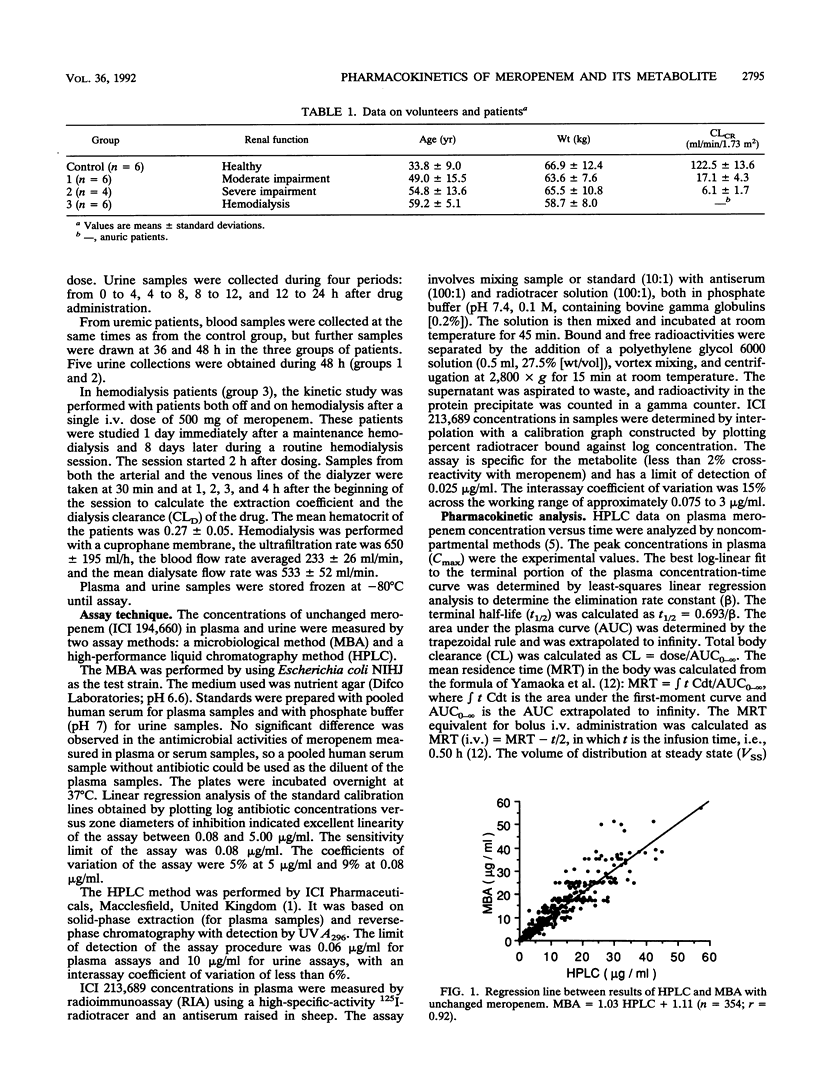
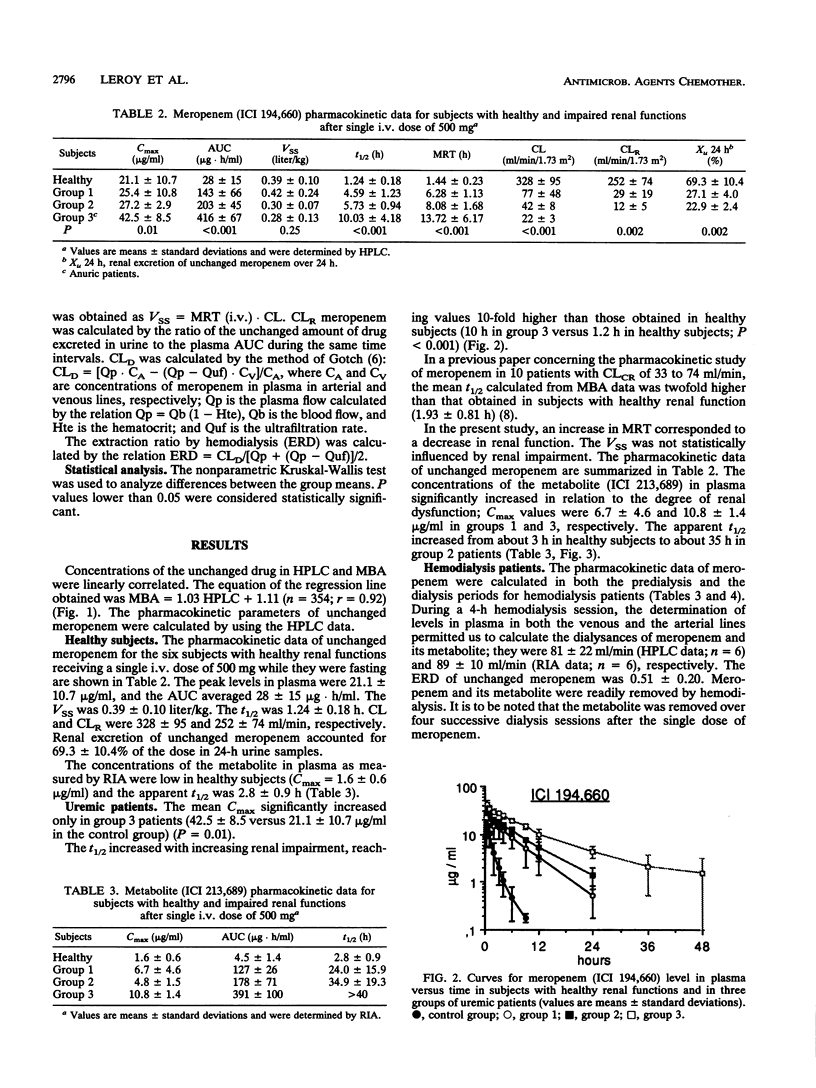
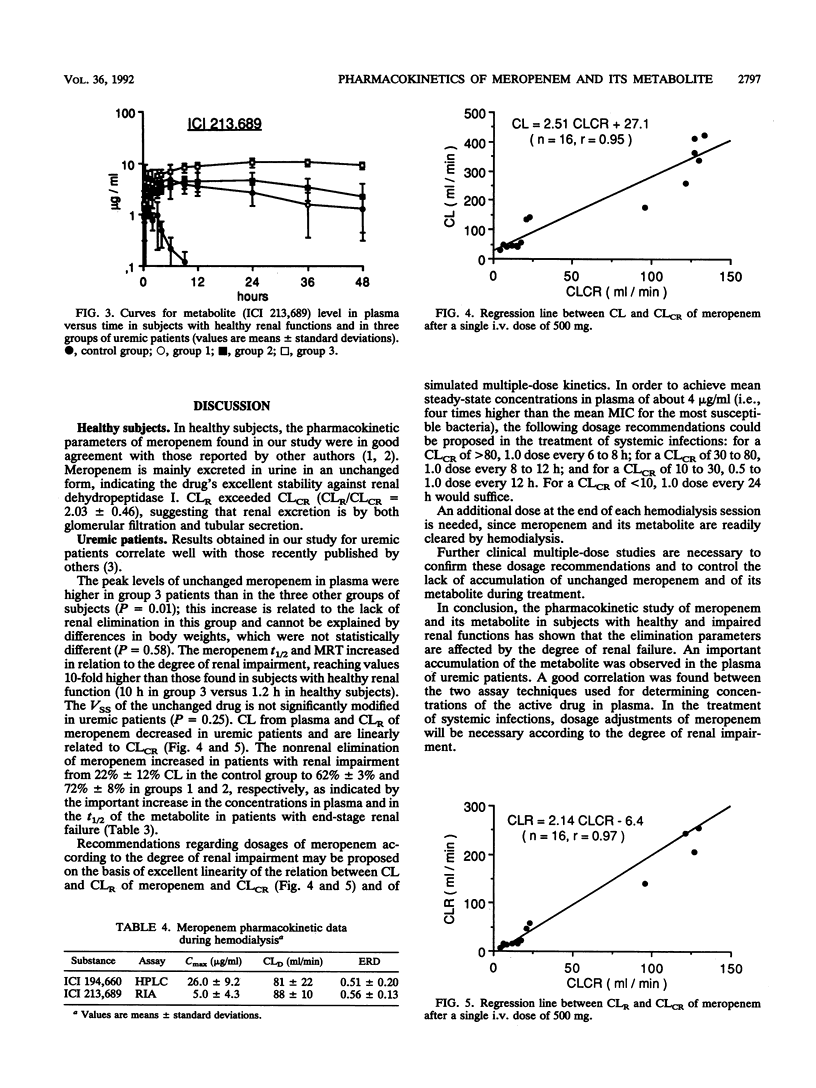
The pharmacokinetics of meropenem (ICI 194,660) and its open-ring metabolite (ICI 213,689) were studied in 6 healthy volunteers and 16 patients with moderate to severe renal impairment after a single intravenous dose of 500 mg given as a 30-min infusion. Concentrations of unchanged meropenem in plasma and urine were measured by both microbiological and high-pressure liquid chromatographic (HPLC) assays. A good correlation was found between the two techniques. Pharmacokinetic parameters of unchanged meropenem were determined by using the HPLC data. The terminal half-life of unchanged meropenem increased in relation to the degree of renal impairment, being 1.2 h in subjects with normal renal function and 10 h in patients with end-stage renal failure. Total body clearance and renal clearance of unchanged meropenem are linearly related to creatinine clearance. The concentrations of the metabolite in plasma, which are very low in healthy subjects, significantly increased in uremic patients. The apparent half-life of ICI 213,689 increased in uremic patients and was about 35 h in patients with severe renal insufficiency. Meropenem and its metabolite are effectively removed by hemodialysis. The dialysis clearance of the unchanged drug was 81 +/- 22 ml/min. Dosage adjustments of meropenem will be necessary in patients with severe renal impairment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax R. P., Bastain W., Featherstone A., Wilkinson D. M., Hutchison M., Haworth S. J. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):311–320. doi: 10.1093/jac/24.suppl_a.311. [DOI] [PubMed] [Google Scholar]
- Burman L. A., Nilsson-Ehle I., Hutchison M., Haworth S. J., Norrby S. R. Pharmacokinetics of meropenem and its metabolite ICI 213,689 in healthy subjects with known renal metabolism of imipenem. J Antimicrob Chemother. 1991 Feb;27(2):219–224. doi: 10.1093/jac/27.2.219. [DOI] [PubMed] [Google Scholar]
- Christensson B. A., Nilsson-Ehle I., Hutchison M., Haworth S. J., Oqvist B., Norrby S. R. Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 1992 Jul;36(7):1532–1537. doi: 10.1128/aac.36.7.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Turner P. J., Wannop C., Withnell E. S., Grindey A. J., Nairn K. In vitro antibacterial activity of SM-7338, a carbapenem antibiotic with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1989 Feb;33(2):215–222. doi: 10.1128/aac.33.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Aldridge K. E., Allen S. D., Barry A. L., Fuchs P. C., Gerlach E. H., Pfaller M. A. Multicenter in vitro evaluation of SM-7338, a new carbapenem. Antimicrob Agents Chemother. 1989 Apr;33(4):562–565. doi: 10.1128/aac.33.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy A., Fillastre J. P., Etienne I., Borsa-Lebás F., Humbert G. Pharmacokinetics of meropenem in subjects with renal insufficiency. Eur J Clin Pharmacol. 1992;42(5):535–538. doi: 10.1007/BF00314864. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Novelli A., Chin N. X. In vitro activity and beta-lactamase stability of a new carbapenem, SM-7338. Antimicrob Agents Chemother. 1989 Jul;33(7):1009–1018. doi: 10.1128/aac.33.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentochnik D. E., Eliopoulos G. M., Ferraro M. J., Moellering R. C., Jr Comparative in vitro activity of SM7338, a new carbapenem antimicrobial agent. Antimicrob Agents Chemother. 1989 Aug;33(8):1232–1236. doi: 10.1128/aac.33.8.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita Y., Inoue M., Mitsuhashi S. In vitro antibacterial activity and beta-lactamase stability of the new carbapenem SM-7338. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):908–916. doi: 10.1007/BF01963782. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978 Dec;6(6):547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]


