Abstract
The goal of this study was to identify the circulating cell that is the immediate precursor of tissue macrophages. ROSA 26 marrow mononuclear cells (containing the β-geo transgene that encodes β-galactosidase and neomycin resistance activities) were cultured in the presence of macrophage colony-stimulating factor and flt3 Ligand for 6 days to generate monocytic cells at all stages of maturation. Expanded monocyte cells (EMC), the immature (ER-MP12+) and more mature (ER-MP20+) subpopulations, were transplanted into irradiated B6/129 F2 mice. β-gal staining of tissue sections from animals 15 min after transplantation demonstrated that the donor cells landed randomly. By 3 h, donor cells in lung and liver were more frequent in animals transplanted with ER-MP20+ (more mature) EMC than in animals transplanted with unseparated EMC or fresh marrow mononuclear cells, a pattern that persisted at 3 and 7 days. At 3 days, donor cells were found in spleen, liver, lung, and brain (rarely) as clusters as well as individual cells. By 7 and 14 days, the clusters had increased in size, and the cells expressed the macrophage antigen F4/80, suggesting that further replication and differentiation had occurred. PCR for the neogene was used to quantitate the amount of donor DNA in tissues from transplanted animals and confirmed that ER-MP20+ EMC preferentially engrafted. These data demonstrate that a mature monocytic cell gives rise to tissue macrophages. Because these cells can be expanded and manipulated in vitro, they may be a suitable target population for gene therapy of lysosomal storage diseases.
Lysosomal storage diseases are inherited disorders caused by the deficiency of enzymes that normally allow the stepwise degradation of metabolic intermediates. The accumulation of these intermediates within lysosomes leads to cellular hypertrophy followed by organ dysfunction (1). Patients present with hepatosplenomegaly, skeletal deformities, and central nervous system abnormalities depending on the enzyme defect. Accumulation of intermediates in several diseases, including Gaucher disease, may be reduced by providing the missing enzyme. Under the principle of cross-correction, the normal enzyme can diffuse across cell and lysosomal membranes to degrade the accumulated metabolic intermediates (2). However, this is a costly and lifelong therapy and it is not uniformly effective (3, 4). Also, neuropathic forms of these diseases may not be palliated by enzyme therapy as the normal enzymes may not cross the blood–brain barrier (1, 4).
Bone marrow transplantation (BMT) is an alternative approach used to reverse visceral abnormalities and stabilize skeletal and neurological dysfunction as tissue macrophages derived from transplanted cells provide a local source of normal enzyme (5–7). Thus, the presence of donor origin Kupffer cells in the liver and macrophages in the spleen leads to the resolution of organomegaly. Microglia, the resident macrophage of the central nervous system, are the effector cells that could lead to stabilization of neurologic defects after transplantation. However, we and others have shown that microglia engraft slowly after BMT (8–10). These kinetics may explain the failure of BMT to stabilize or improve neurologic symptoms in many patients.
Recent murine studies have shown that stem cell gene therapy can decrease lysosomal storage in the liver and spleen (11–13). However, as proviral integration requires cell division and stem cells are quiescent, transduction rates by retroviral vectors are low. If the circulating precursor of tissue macrophages could be identified, it might be a nonquiescent, easily manipulated population suitable for transplantation and as a target cell population for gene therapy for lysosomal storage diseases.
The traditional view of the mononuclear phagocyte system is that monocytes develop in the bone marrow and are released into the circulation from where they enter tissues to become resident macrophages (14, 15). A number of studies suggest that this view may not be entirely correct. Prior studies have shown that tissue macrophages may divide in situ, leading to renewal of this population (16–18). An intriguing possibility is that a less mature marrow-derived cell, such as a macrophage colony-forming unit (CFU-M), a less mature marrow-derived cell, enters tissues and differentiates into macrophages (19).
Our goal was to identify the murine macrophage precursor cell that engrafts at tissue sites and differentiates into Kupffer cells, alveolar macrophages, and microglia. We previously have studied the kinetics of macrophage engraftment by using a ROSA 26 transplantation model (8). Cells from this transgenic mouse contain the β-geo gene, which encodes for β-galactosidase (β-gal) and neomycin resistance (neo) (20, 21). A strength of this model is that individual ROSA 26-derived cells can be identified on a background of recipient cells by histochemical staining of tissues for β-gal. For the current studies, we cultured ROSA 26 bone marrow mononuclear cells in the presence of both macrophage colony-stimulating factor (M-CSF) and flt3 Ligand (FL) to generate a population of cells committed to monocytic differentiation. Expanded precursors then were separated into early- and late-maturation-stage populations by using the surface markers ER-MP12 and ER-MP20 and transplanted into recipient mice. The results of our studies demonstrate that a more mature monocytic cell, expressing the ER-MP20 marker, engrafts (i.e., lands, replicates, and differentiates) at tissue sites.
MATERIALS AND METHODS
Animals.
ROSA 26 breeding pairs were a gift from Philipe Soriano (Fred Hutchinson Cancer Research Center, Seattle, WA). Animals 6 to 10 weeks of age were used as donors for BMT. The use of the ROSA 26 mouse and B6/129 F2 recipient mice have been described previously (8).
Preparation of BM.
Marrow was prepared from ROSA 26 donor mice as described previously (8). Fresh mononuclear cells were prepared by separation on an LSM density gradient (Organon Teknika–Cappel) at 400 × g for 20 min. Mononuclear cells at the interface were removed carefully, washed twice, and were suspended at 107 cells/ml before transplantation.
Mouse Transplantation Protocol.
Beginning 2 weeks before transplantation, B6/129 F2 mice were given drinking water containing Baytril (Bayer, Elkhart, IN), 5 ml/200 ml of acidified tap water. Immediately before transplantation, mice were placed in a pie-shaped restrainer and received 1,050 cGy radiation from a dual-Cesium source. Recipient mice then received 5 × 106 fresh marrow mononuclear cells or expanded monocytic cells (EMC) via tail-vein injection. Transplanted animals continued to receive antibiotics for 2–3 weeks after transplantation. Three animals were studied at each time point with each population of transplanted cells.
β-Gal Staining.
Frozen tissue sections were prepared, stained, and analyzed to detect β-gal+ cells as described previously (8). Sections were counterstained with Mayer’s Hematoxylin before permanent mounting.
Hematopoietic Colony Assays.
Freshly isolated marrow mononuclear cells or EMC were examined for production of hematopoietic colonies as described previously (8). For enumeration of CFU-M, recombinant human M-CSF (25 units/ml) (Genzyme) was added to each plate. Colonies (>50 cells) were counted using an inverted microscope and expressed as CFU-M per 2 × 105 marrow mononuclear cells.
Preparation of EMC.
Fresh marrow mononuclear cells were prepared as described above and placed at a density of 106/ml in Iscove’s modified Dulbecco’s medium supplemented with 20% FCS, 1% antibiotic-antimycotic (GIBCO/BRL), M-CSF (25 units/ml), and recombinant human FL (100 ng/ml, provided by Immunex). The cells were incubated in 75-cm2 flasks (Costar) for 6 days in a humidified incubator containing 5% CO2 at 37°C.
mAbs.
mAbs directed against the murine markers F4/80, ER-MP12, ER-MP20, and ER-MP58 were purchased from BMA Biomedicals. Isotype controls were purchased from PharMingen.
Separation of Cell Populations Using Magnetic Cell Sorting.
Fresh marrow mononuclear cells and EMC were separated by initially incubating the cells with mAbs directed against subpopulations of interest. Cells were prepared by washing and resuspension in PBS with 5 mM EDTA and 1% BSA (Sigma). Two to 5 μl of antibody supernatant was added for each 106 cells and incubated on ice for 30 min. The cells were washed once and resuspended in a small amount of buffer. Goat anti-rat IgG labeled with magnetic microbeads (20 μl/107 cells, Miltenyi Biotec, Auburn, CA) was added for a further 30 min on ice. The cells then were diluted with a small amount of buffer and layered over a prewashed magnetic wool column placed in a VarioMacs (Miltenyi Biotec). The cells were allowed to pass through the column and collected followed by 3 washes with ice-cold buffer. These cells were pooled as the antibody-negative population. The column was washed with buffer to remove nonspecifically adhering cells and then removed from the magnet, allowing elution of antibody-positive cells. The purity of the positive and negative cell populations was confirmed by incubating 106 cells with phycoerythrin-conjugated anti-rat IgG (H+L) (Southern Biotechnology Associates) (1:50 dilution in 100 μl for 30 min on ice). These cells were then washed three times and analyzed by using flow cytometry (Coulter). Positive and negative cell populations were 90% pure or greater.
Immunocytochemistry.
Immunocytochemical staining of β-gal-stained tissue sections to detect the F4/80 antigen was performed as described previously (8).
PCR Analysis.
Genomic DNA first was isolated from tissues (bone marrow, liver, spleen, lung, and brain) by extraction from homogenized tissues (22). One piece of tissue, generally 100–200 mg in weight, was taken from each of three animals studied at each time point. One thousand nanograms of purified DNA from tissues was added to Pfu polymerase, buffer, and deoxynucleotide triphosphates as specified in the Pfu polymerase kit (Stratagene). These samples then were added to PCR tubes containing a set of primers for the neo gene or primers for GAPDH (manufactured by GIBCO/BRL). Neo primers were 5′-CAC TGA AGC GGG AAG GGA CTG and 5′-CAA TAT CAC GGG TAG CCA ACG. GAPDH primers were 5′-CCA TGG AGA AGG CTG GGG and 5′-CAA AGT TGT CAT GGA TGA. Amplifications were at 95°C for 3 min, 95°C for 1 min, 60°C for 1.5 min, and 72°C for 2 min, with 25 cycles for initial neo amplification and 25 cycles for GAPDH amplification followed by a final 10-min extension at 72°C for both reactions. In some cases the initial neo PCR products were further amplified by using a set of nested neo primers by addition of 10 μl of the initial reaction product to a second reaction mixture. The neo PCR conditions for the second reaction were identical to those described for the initial product with the exception of the addition of a [32P]dCTP to the reaction mix. The expected band size was 434 bp for the neo product and 195 bp for the GAPDH product. Negative controls in each reaction set included no DNA and DNA extracted from B6/129 F2 mice. Positive controls include a standard curve of serial dilutions of DNA from ROSA 26 mice diluted in DNA extracted from B6/129 F2 mice. Unlabeled products were separated on 1.2% agarose gels. Band intensity of ethidium bromide stained gels was compared with the standard curve generated. The 32P-labeled amplified products were separated on 8% polyacrylamide gels. Image analysis was performed by using whole band analyzer software. Using these techniques, neo-containing sequences in transplanted animals were detectable to the range of 0.001–0.0001% ROSA DNA.
Statistical Analysis.
All statistical analyses were performed by the Student’s t test.
RESULTS
M-CSF and FL Stimulation of Marrow Mononuclear Cells Leads to Expansion of CFU-M.
M-CSF and FL have been shown to synergize in inducing macrophage colony formation by progenitor cells present in peripheral blood and cord blood. To investigate whether this combination of growth factors would increase the number of CFU-M, marrow mononuclear cells from ROSA 26 mice were placed in suspension culture with M-CSF (25 units/ml) and FL (100 ng/ml). At various time points, cells were removed and cultured on agar in the presence of M-CSF to enumerate CFU-M. Maximal expansion (60- to 80-fold) of CFU-M (cell number multiplied by CFU-M/2 × 105 cells) was seen on days 6–7 in all studies (data not shown). Thus, for subsequent studies of EMC, day 6 of culture with M-CSF and FL was chosen for analysis. More than 95% of day 6 EMC were nonspecific esterase positive (weak + strong staining). The population was composed of cells committed to the monocytic lineage at all stages of differentiation (see below). At this time the total number of cells per culture was 1–2× the input number.
Antigen Expression by Fresh Marrow Mononuclear Cells and EMC.
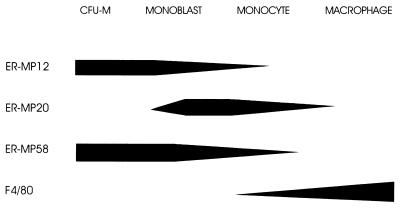
Previous studies have shown that the early myeloid differentiation antigen ER-MP12 is present on 6–9% of BM cells. The marker ER-MP20 is present on only 4–6% of BM cells in a brightly staining fashion, but on 31–43% in a dull staining pattern (23). Fig. 1 shows a diagrammatic representation of the expression of these antigens during monocyte and macrophage differentiation. We analyzed the expression of these markers on freshly isolated marrow mononuclear cells and EMC. By day 6, 95% of EMC expressed the marker of M-CSF responsiveness, ER-MP58 (24), as determined by FACS analysis. Thus, as the cells were committed to a monocytic lineage, ER-MP12 and ER-MP20 could be used as differentiation antigens. ER-MP12 was expressed by 40–50% of the EMC population (dull + bright staining) while 30–50% of EMC expressed ER-MP20 (dull + bright staining).
Figure 1.
Diagram of antigen expression during monocyte and macrophage differentiation. Adapted in part from ref. 23.
Landing and Engraftment of Fresh Marrow Mononuclear Cells and EMC.
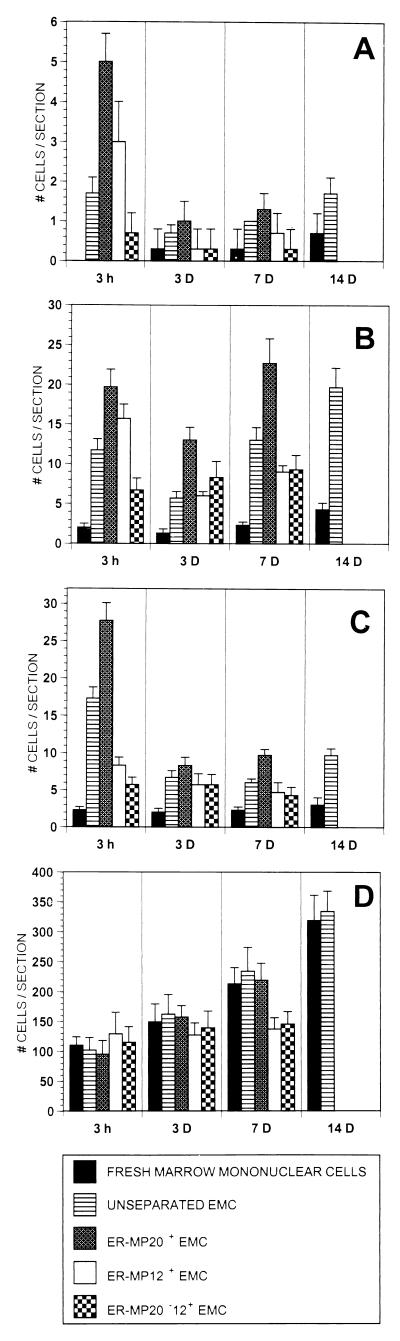
Fresh marrow mononuclear cells or EMC (5 × 106) were transplanted into irradiated B6/129F2 hosts. Tissues from these transplanted animals were examined for the presence of donor cells by examination of β-gal-stained frozen tissue sections. At 15 min after transplantation, the landing of both fresh marrow mononuclear cells and EMC appeared to be random. Within individual tissues, some tissue sections had no β-gal-positive cells, while others had a number of cells (data not shown). A consistent finding was the absence of any donor cells in the brains of transplanted animals at 15 min. By 3 h after transplantation, numerous cells were seen in the spleens of animals transplanted with both fresh marrow mononuclear cells and EMC. There appeared to be no preferential landing in the spleen by unseparated EMC (Fig. 2). In contrast, unseparated EMC were seen preferentially in the lung, liver, and brain of transplanted animals by 3 h. As can be seen in Fig. 2, there was an average of 11.7 donor cells per tissue slice in the lung and 17.3 donor cells in the liver of animals transplanted with EMC. This is in contrast to averages of 2.0 and 2.3 donor cells per tissue slice, respectively, in the lung and liver of animals transplanted with fresh marrow mononuclear cells (P < .005, in both cases).
Figure 2.
β-gal+ donor cell landing in brain (A), lung (B), liver (C), and spleen (D) after transplantation. Fresh marrow mononuclear cells, EMC, or EMC fractionated into ER-MP20+, ER-MP12+, and ER-MP20−12+ subpopulations were transplanted into B6/129F2 mice. Organs were removed at various time points and frozen tissue sections were prepared and studied for the presence of β-gal+ cells. Data are presented as the average number of β-gal+ cells per tissue section (y axis).
As described above, day 6 EMC are a heterogeneous population of cells. To analyze whether one of the subpopulations was more efficient at landing at tissue sites, EMC were fractionated by using magnetic cell sorting separation techniques. Day 6 cultures of EMC were pooled and positively selected for ER-MP12+ cells (early macrophage lineage) or ER-MP20+ cells (later macrophage lineage). Some cells in this heterogeneous population were both ER-MP12+ and ER-MP20+. Therefore, ER-MP20− cells subsequently were positively selected for the marker ER-MP12 to purify an ER-MP20−12+ population. Fig. 2 shows that ER-MP20+ EMC are seen at tissue sites more frequently at 3 h after transplantation than are fresh marrow mononuclear cells, unfractionated EMC, or ER-MP20−12+ EMC.
By 3 days after transplantation, the number of donor cells in all tissue sections other than spleen had declined. This may reflect the death of donor cells that landed but failed to engraft or perhaps the redistribution of donor cells to the spleen. Three additional observations were clear at this time point. First, EMC-derived cells clearly were present more frequently than fresh marrow mononuclear cells at tissue sites (P < .01 brain, P < .005 liver, lung) (Fig. 2). Second, donor EMC and fractionated EMC-derived cells appeared to be present at tissue sites in small clusters of cells. This was most notable in the spleen but was also seen in the liver and lung (Fig. 3A). A third finding at this time point was that ER-MP20+ EMC-derived cells were present more frequently at tissue sites than cells derived from unfractionated EMC, ER-MP12+ EMC, or ER-MP20−12+ EMC (Fig. 2).
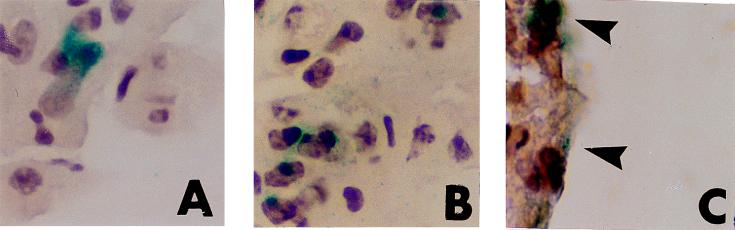
Figure 3.
Engraftment of expanded monocytic cells in lung and brain after transplantation. β-Gal staining of cryostat sections of tissues from B6/129F2 animals transplanted with M-CSF- and FL-expanded ROSA 26 macrophage precursors are shown. A and B are sections from lung demonstrating a doublet of β-gal+ (blue) cells 3 days after transplantation (A) and a larger cluster of β-gal+ cells at 7 days (B). Rare groups of β-gal+ cells were seen in the brain of an animal at 14 days (C, arrows).
At 7 days after transplantation the average number of EMC-derived cells per tissue slice had increased in the lung but not in the spleen, brain, or liver. ER-MP20+-derived cells had also increased in number in the lung but not in other tissues. EMC-derived cells continued to be seen more frequently at tissue sites than cells derived from transplanted fresh marrow mononuclear cells (P < .005). In addition, ER-MP20+ EMC-derived cells were clearly distributed more frequently at all tissue sites compared with ER-MP12+ and ER-MP20−12+ EMC-derived cells (Fig. 2).
None of the animals transplanted with 5 × 106 fractionated EMC survived to 14 days after transplantation (presumably reflecting the absence of donor hematopoietic stem cells). However, as shown in Fig. 2, unfractionated EMC-derived cells continued to be found at tissue sites in the brain, lung, and liver more frequently (2–3×) than fresh marrow mononuclear cell-derived cells.
Clusters Begin by 3 Days After Transplantation.
Donor cells were present at 15 min and 3 h after transplantation as single cells in spleen, lung, liver, and brain. However, by 3 days after transplantation, clusters of cells could be seen in animals transplanted with EMC (both fractionated and unfractionated). Clusters with 4–16 donor cells were identified in the spleen while doublets of cells were seen in the liver and lung (Fig. 3A). Clusters were more frequent by 7 days at all sites and had increased in size. As seen in Fig. 3B larger clusters (i.e., 4–8 cells) were present in the lung by 7 days after transplantation.
In contrast to the findings in the spleen, lung, and liver, engraftment of cells in the brain was low. Only rare ROSA 26 origin (β-gal+) cells were seen in the brains of animals transplanted with any of the cell populations. However, groups of cells were seen at leptomeningeal sites in the brains of animals transplanted with EMC, consistently by 7–14 days after transplantation (Fig. 3C). This was not seen in animals transplanted with fresh marrow mononuclear cells.
EMC-Derived Cells Express the F4/80 Antigen by 7 Days After Transplantation.
Immunocytochemical staining was performed on frozen sections from liver, lung, and brain taken from animals transplanted with unseparated EMC. At 3 h after transplantation the percentage of donor cells expressing the F4/80 macrophage differentiation antigen (25) was low: 0% in the brain, 9% in the lung, and 14% in the liver. By 3 days this had increased to 67% in the brain, 20% in the lung, and 28% in the liver. Moreover, by 7 days the number of F4/80+ donor cells had increased to 100% in the brain, 70% in the lung, and 75% in the liver, indicating that macrophage differentiation was occurring in the EMC-derived cells that had engrafted.
PCR Analysis of Tissues After Transplantation.
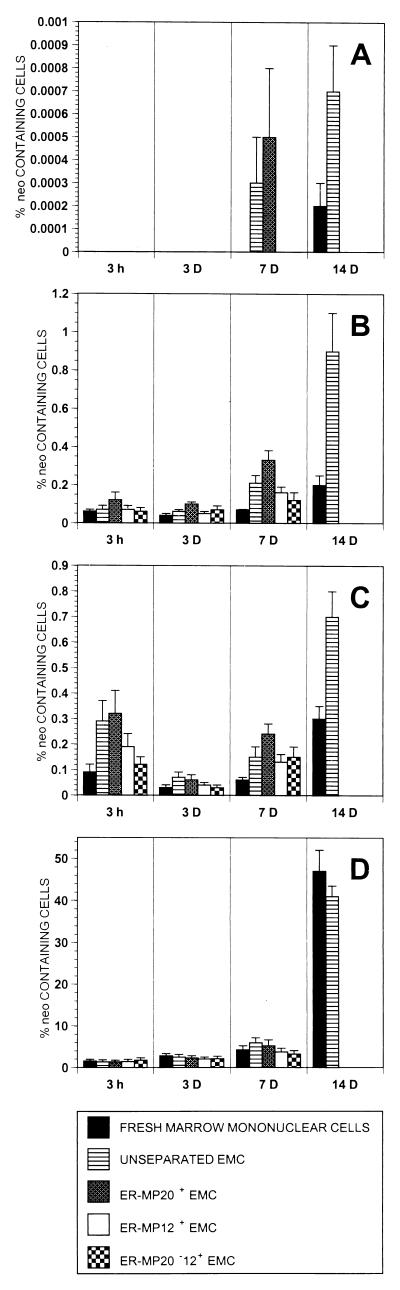
To confirm the landing and engraftment data obtained from analysis of β-gal-stained tissue sections as well as quantitate amounts of donor cell DNA in tissue specimens, we performed semiquantitative PCR analysis for the neo gene on genomic DNA prepared from tissue samples from the same transplanted B6/129 F2 mice. At 3 h after transplantation, high levels of neo-containing cells (1.3–1.7%) were seen in the spleens of mice transplanted with both fresh marrow mononuclear cells and EMC. No neo DNA was detectable in the brains of these animals, indicating that there was less than 0.0001% donor cells present. By 3 h EMC were more frequent in brain, lung, and liver than fresh marrow mononuclear cells, and ER-MP20+ EMC clearly were more frequent than ER-MP20−12+ EMC (Fig. 4).
Figure 4.
neo+ donor cell landing in various brain (A), lung (B), liver (C), and spleen (D) after transplantation. Fresh marrow mononuclear cells, EMC, or EMC fractionated into ER-MP20+, ER-MP12+, and ER-MP20−12+ subpopulations were transplanted into B6/129F2 mice. Organs were removed at various time points, and genomic DNA was extracted and studied for the presence of neo+ cells by using a semiquantitative PCR. Data are presented as the average percentage of neo+ cells per tissue sample (y axis).
At 3 days the differences between the groups lessened, as was seen in the β-gal-stained tissue sections. Engraftment of ROSA 26 cells in the spleen increased about 2-fold in all groups by PCR assay for the neo sequence. ER-MP20+ EMC engrafted better in the lung and liver at this time point; however, the differences were small (Fig. 4).
By 7 days after transplantation, donor cells had increased in the spleen to levels as high as 5.9% in the unseparated EMC group. ER-MP20+ EMC-derived cells comprised 5.2% of cells in the spleen, compared with 3.7% for the ER-MP12+ EMC and 3.2% for ER-MP20−12+ EMC-derived cells. At this time point neo-bearing cells were detectable at low levels in the brains of mice transplanted with unfractionated EMC and ER-MP20+ EMC. The frequency of ROSA 26 ER-MP20+ EMC-derived cells had increased to 0.33% in the lung versus 0.07% in the fresh marrow mononuclear cell group and 0.24% in the liver versus 0.06% in the fresh marrow mononuclear cell group. ER-MP20+ EMC-derived cells were more frequent than ER-MP20−12+ EMC-derived cells at 7 days in the lung (0.33% versus 0.12%) and in the liver (0.24% versus 0.15%) (Fig. 4).
By 14 days after transplantation of fresh marrow mononuclear cells and EMC there were high levels of engraftment in the spleen: 47% and 41%, respectively. At all other tissue sites studied, EMC engrafted at two to four times the levels seen in the mice transplanted with fresh marrow mononuclear cells (Fig. 4).
These results from PCR analyses confirmed the β-gal-staining studies, showing that EMC engrafted at tissue sites more rapidly and at higher levels than fresh marrow mononuclear cells. In addition, ER-MP20+ EMC appeared to engraft at higher levels than ER-MP20−12+ EMC.
We transplanted six additional mice, three with fresh marrow mononuclear cells and three with ER-MP20+ fresh marrow mononuclear cells, and studied their tissues for engraftment of neo-bearing cells 7 days after transplantation by using semiquantitative PCR. The average percentage of neo-containing cells in spleen, lung, and liver was 4.5, 0.1, and 0.08 in animals transplanted with fresh marrow mononuclear cells and 5.7, 0.3, and 0.1 in animals transplanted with ER-MP20+ fresh marrow mononuclear cells, suggesting an engraftment advantage in the lung and liver for ER-MP20+ fresh marrow mononuclear cells. The small differences between these groups may reflect the fact that fresh marrow mononuclear cells are approximately 40% ER-MP20+, and thus the ER-MP20+ fresh marrow mononuclear cells are enriched only about 2-fold over fresh marrow mononuclear cells. [ER-MP20 marks endothelial cells and some lymphocytes (26) that would be present in the fresh marrow mononuclear cell population.] The EMC population used in earlier studies is devoid of contaminating ER-MP20+ nonmonocytic cells.
DISCUSSION
The goal of these studies was to identify the murine macrophage precursor cell that engrafts at tissue sites to give rise to alveolar macrophages, Kupffer cells, and microglia. Previous data from mouse and human studies have demonstrated that these cells are marrow-derived (14, 15) but the stage of differentiation of the immediate precursor cell had not been defined. To generate a large population of cells committed to monocytic lineage, we chose to use the synergistic combination of M-CSF and FL (27–34). We found that after 6 days of culture, the number of CFU-M were maximally expanded. In addition, >95% of the cells expressed the ER-MP58, a marker that correlates with M-CSF responsiveness. We then chose to separate these EMC into populations expressing immature and more mature monocytic precursor antigens.
The mAb against ER-MP12 recognizes early-lineage hematopoietic cells (ref. 35; Fig. 1). The ER-MP12 antigen recently has been identified as the vascular endothelial adhesion molecule PECAM-1 (CD31) (36). It is expressed on long-term repopulating cells, CD34+ cells, and early myeloid and early B cells (37). ER-MP12 expression increases on maturation toward colony-forming units in culture. Separate domains on the PECAM-1 molecule have been shown to be involved in the migration of monocytes across endothelium and the passage of monocytes through the extracellular matrix (38). Phosphorylation of PECAM-1 has been shown to induce migration of monocytes (39).
The marker ER-MP20 is expressed on more mature monocytic cells (23, 25, 40), but falls with differentiation to macrophages (see Fig. 1). The ER-MP20 antigen is identical to the murine antigen Ly-6C, a monocyte/macrophage and endothelial cell-differentiation antigen regulated by interferon-γ (41). Ly-6C also has been shown to regulate endothelial adhesion and homing of T cells by activating integrin-dependent adhesion pathways (42). Thus, in addition to identifying different maturation stages of monocytic cells, ER-MP12 and ER-MP20 recognize determinants functionally important in adhesion and migration.
We found that both fresh marrow mononuclear cells and EMC land randomly at 15 min after transplantation. This suggests that initial distribution may be a result of blood flow to various organs and independent of the phenotype of the cell. However, by 3 h after transfer there was an increased number of EMC in brain, lung, and liver. This implies that populations of cells enriched for the monocytic lineage preferentially enter tissues or are selectively retained there (after random landing). By 3 days, the number of β-gal+ donor cells in the tissues declined as there may be donor cell death or redistribution of cells during the interval from 3 h to 3 days. However, clusters of donor cells were also apparent at this time, indicating that cell replication occurred within tissue sites. By 7 days after transplantation, the number and size of these clusters had increased. Since these cells were shown to be F4/80+, donor cells had engrafted (entered tissue sites, replicated, and differentiated). It is possible that mature macrophages had phagocytosed dying donor (β-gal+) cells, thus giving the appearance of donor cells expressing the F4/80 antigen. However, given that our PCR data demonstrate an increase in donor cell (neo-containing) DNA at 7 days, this seems unlikely.
At all time points, ER-MP20+ EMC preferentially landed in brain, liver, and lung, indicating an engraftment advantage for these more mature monocytic cells over the less mature ER-MP12+ and ER-MP20−12+ populations. The engraftment of ER-MP12+ EMC is intermediate to ER-MP20+ and ER-MP20−12+ EMC. This may reflect the presence of double-positive ER-MP20+12+ cells (more mature phenotype) in the ER-MP12+ EMC population.
We used PCR as an alternative method to quantitate the percentage of donor-derived (neo-containing) cells in tissue samples from the same animals used in the β-gal studies. These studies confirm the findings of the β-gal staining, namely, that EMC preferentially land and engraft at tissue sites and that ER-MP20+ EMC engraft at higher levels than ER-MP20−12+ EMC. A recent report has demonstrated that peritoneal macrophages can be transplanted successfully into mice affected by MPS VII (43). These macrophages were detectable in tissues for as long as 1 month and resulted in a reduction in lysosomal storage. While there was no suggestion of cell replication (i.e., clusters of donor cells could not be identified), these data support the idea that a cell with a more mature monocytic phenotype can enter and persist at tissue sites.
Previous studies have suggested that the process of in vitro expansion of murine marrow cells in the presence of cytokines leads to a stem cell engraftment defect (44). Our results show that a 6-day culture of marrow mononuclear cells in the presence of M-CSF and FL results in an expansion of macrophage precursor cells and a preservation (and perhaps enhancement) of their engrafting ability. Further characterization of this cell may lead to identification of a target cell population that would be suitable for gene therapy for lysosomal storage diseases. In a broader sense, these cells may serve as vehicles for the delivery of genes, drugs, or cellular activities to the spleen, liver, lung, and central nervous system.
Macrophage repopulation after transplantation occurred rapidly in the spleen where radiation depletes local macrophages. However, host macrophages in other tissues persisted after radiation as numerous F4/80+,β-gal− cells were seen in brain, lung, and liver at 7 days (unpublished observations). Key to successful clinical applications is a better understanding of the mechanisms responsible for the turnover of macrophages at different tissue sites.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK 49652 and K08 DK 02443. J.L.A. is the recipient of a Faculty Research Award from the American Cancer Society. D.W.K. is the recipient of a fellowship from the Cure for Lymphoma Foundation.
ABBREVIATIONS
- EMC
expanded monocytic cells
- FL
flt3 Ligand
- M-CSF
macrophage colony-stimulating factor
- β-gal
β-galactosidase
- neo
neomycin-resistance gene
- CFU-M
macrophage colony-forming unit
References
- 1.Neufeld E F, Muenzer J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2465–2494. [Google Scholar]
- 2.Braun S E, Aronovich E L, Anderson R A, Crotty P L, McIvor R S, Whitley C B. Proc Natl Acad Sci USA. 1993;90:11830–11834. doi: 10.1073/pnas.90.24.11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabowski G A, Barton N W, Pastores G, Dambrosia J M, Banerjee T K, McKee M A, Parker C, Schiffmann R, Hill S C, Brady R O. Ann Intern Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Kaye E M. Curr Opin Pediatr. 1995;7:650–654. doi: 10.1097/00008480-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Resnick J M, Krivit W, Snover D C, Kersey J H, Ramsay N K C, Blazar B R, Whitley C B. Bone Marrow Transplant. 1992;10:273–280. [PubMed] [Google Scholar]
- 6.Tsai P, Lipton J M, Sahdev I, Najfeld V, Rankin L R, Slyper A H, Ludman M, Grabowski G A. Pediatr Res. 1992;31:503–507. doi: 10.1203/00006450-199205000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Krivit W, Shapiro E G, Peters C, Wagner J E, Cornu G, Kurtzberg J, Wenger D A, Kolodny E H, Vanier M T, Loes D J, et al. N Engl J Med. 1998;338:1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy D W, Abkowitz J L. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 9.Krall W J, Challita P M, Perlmutter L S, Skelton D C, Kohn D B. Blood. 1994;83:2737–2748. [PubMed] [Google Scholar]
- 10.Lassmann H, Schmied M, Vass K, Hickey W F. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- 11.Sands M S, Wolfe J H, Birkenmeier E H, Barker J E, Vogler C, Sly W S, Okuyama T, Freeman B, Nicholos A, Muzyczka N, et al. Neuromusc Disord. 1997;7:352–360. doi: 10.1016/s0960-8966(97)00061-8. [DOI] [PubMed] [Google Scholar]
- 12.Correll P H, Colilla S, Dave H P G, Karlsson S. Blood. 1992;80:331–336. [PubMed] [Google Scholar]
- 13.Ohashi T, Boggs S, Robbins P, Bahnson A, Patren K, Wei F-S, Wei J-F, Li J, Lucht L, Fei Y, et al. Proc Natl Acad Sci USA. 1992;89:11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crofton R W, Diesselhoff-den Dulk M M C, van Furth R. J Exp Med. 1978;146:1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas E D T, Ramberg R E, Sale G E, Sparkes R S, Golde D W. Science. 1976;192:1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Naito M, Moriyama H, Umezu H, Matsuo H, Kiwada H, Arakawa M. Am J Pathol. 1996;149:1271–1286. [PMC free article] [PubMed] [Google Scholar]
- 17.Wijffels J F A M, Hendricks R J B M, Steenbergen J J E, Eestermans I L, Beelen R H J. Res Immunol. 1992;143:401–409. doi: 10.1016/s0923-2494(05)80072-0. [DOI] [PubMed] [Google Scholar]
- 18.Lawson L J, Perry V H, Gordon S. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Naito M, Takeya M. Pathol Int. 1996;46:473–485. doi: 10.1111/j.1440-1827.1996.tb03641.x. [DOI] [PubMed] [Google Scholar]
- 20.Freidrich G, Soriano P. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 21.Zambrowicz B P, Imamoto A, Fiering S, Herzenberg L A, Kerr W G, Soriano P. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chomczynski P, Mackey K, Drews R, Wilfinger W. BioTechniques. 1997;22:550–553. doi: 10.2144/97223pf01. [DOI] [PubMed] [Google Scholar]
- 23.Leenen P J M, Melis M, Slieker W A T, Van Ewijk W. Eur J Immunol. 1990;20:27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- 24.de Bruijn M F T R, Ploemacher R E, Mayen A E M, Slieker W A T, van Ewijk W, Leenen P J M. Eur J Immunol. 1996;26:2850–2858. doi: 10.1002/eji.1830261208. [DOI] [PubMed] [Google Scholar]
- 25.Austyn J M, Gordon S. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 26.Leenen P J M, de Bruijn M F T R, Voerman J S A, Cambell P A, van Ewijk W. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 27.Cecchini M G, Hofstetter W, Halasy J, Wetterwald A, Felix R. Mol Reprod Dev. 1997;46:75–84. doi: 10.1002/(SICI)1098-2795(199701)46:1<75::AID-MRD12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Rohrschneider L R, Bourette R P, Lioubin M N, Algate P A, Myles G M, Carlberg K. Mol Reprod Dev. 1997;46:96–103. doi: 10.1002/(SICI)1098-2795(199701)46:1<96::AID-MRD15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Rappold I, Ziegler B L, Kohler I, Marchetto S, Rosnet O, Birnbaum D, Simmons P J, Zannettino A C W, Hill B, Neu S, et al. Blood. 1997;90:111–125. [PubMed] [Google Scholar]
- 30.Gabbianelli M, Pelosi E, Montesoro E, Valtieri M, Luchetti L, Samoggia P, Vitelli L, Barberi T, Testa U, Lyman S, Peschle C. Blood. 1995;86:1661–1670. [PubMed] [Google Scholar]
- 31.Broxmeyer H E, Lu L, Cooper S, Ruggieri L, Li Z-H, Lyman S D. Exp Hematol. 1995;23:1121–1129. [PubMed] [Google Scholar]
- 32.Lyman S D, James L, Bos T V, de Vries P, Brasel K, Gliniak B, Hollingsworth L T, Picha S, McKenna H J, Splett R R, et al. Cell. 1993;75:1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 33.Ohishi K, Katayama N, Itoh R, Mahmud N, Miwa H, Kita K, Minami N, Shirakawa S, Lyman S D, Shiku H. Blood. 1996;87:1718–1727. [PubMed] [Google Scholar]
- 34.Namikawa R, Muench M O, Roncarolo M G. Stem Cells. 1996;14:388–395. doi: 10.1002/stem.140388. [DOI] [PubMed] [Google Scholar]
- 35.van der Loo J C M, Slieker W A T, Kieboom D, Ploemacher R E. Blood. 1995;85:952–962. [PubMed] [Google Scholar]
- 36.Ling V, Luxenberg D, Wang J, Nickbard E, Leenen P J M, Neben S, Kobayasi M. Eur J Immunol. 1997;27:509–514. doi: 10.1002/eji.1830270223. [DOI] [PubMed] [Google Scholar]
- 37.Watt S M, Williamson J, Genevier H, Fawcett J, Simmons D L, Hatzfeld A, Nesbitt S A, Coombe D R. Blood. 1993;82:2649–2663. [PubMed] [Google Scholar]
- 38.Liao F, Huynh H K, Eiroa A, Greene T, Polizzi E, Muller W A. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalra V K, Shen Y, Sultana C, Rattan V. Am J Physiol. 1996;271:H2025–H2034. doi: 10.1152/ajpheart.1996.271.5.H2025. [DOI] [PubMed] [Google Scholar]
- 40.de Bruijn M F T R, Slieker W A T, van der Loo J C M, Voerman J S A, van Ewijk W, Leenen P J M. Eur J Immunol. 1994;24:2279–2284. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- 41.Jutila M A, Kroese F G M, Jutila K L, Stall A M, Fiering S, Herzenberg L A, Berg E L, Butcher E C. Eur J Immunol. 1988;18:1819–1826. doi: 10.1002/eji.1830181125. [DOI] [PubMed] [Google Scholar]
- 42.Hanninen A, Jaakkola I, Salmi M, Simell O, Jalkanen S. Proc Natl Acad Sci USA. 1997;94:6898–6903. doi: 10.1073/pnas.94.13.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman B, Vogler C, Hofling A, Nicholes A, Sands M. Blood. 1997;90:120a. (abstr.). [PubMed] [Google Scholar]
- 44.Peters S O, Kittler E L W, Ramshaw H S, Quesenberry P J. Blood. 1996;87:30–37. [PubMed] [Google Scholar]