Abstract
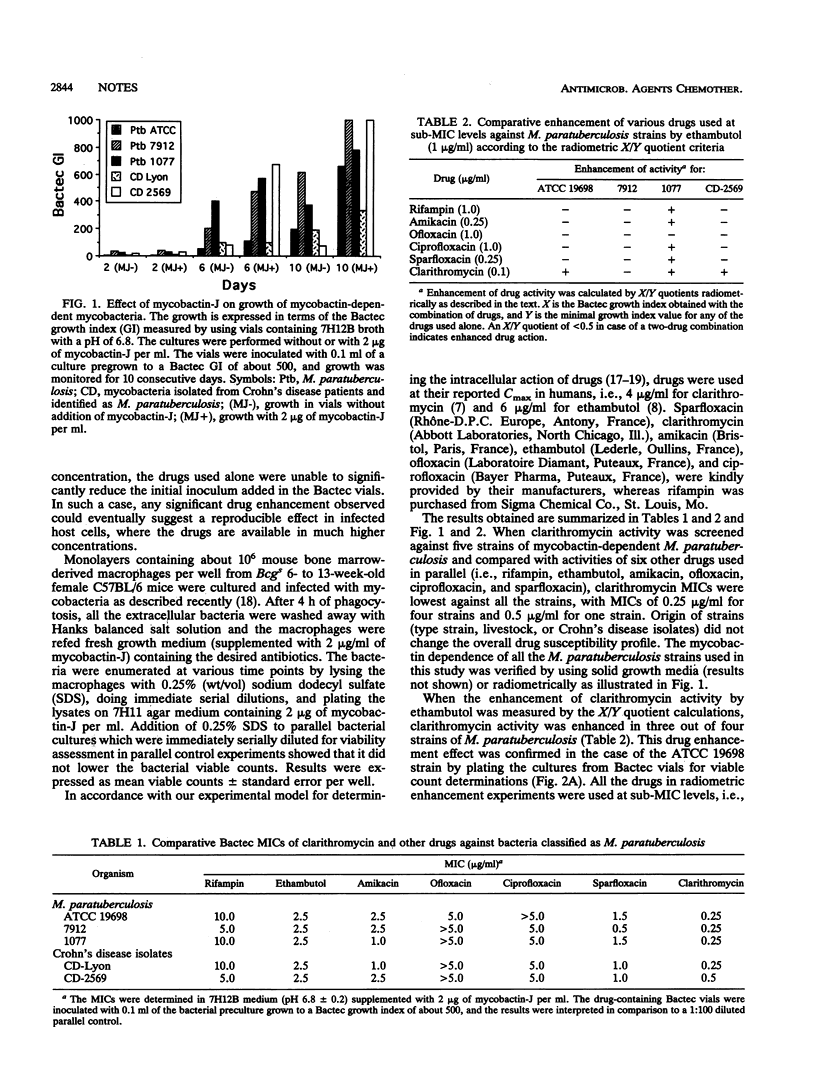
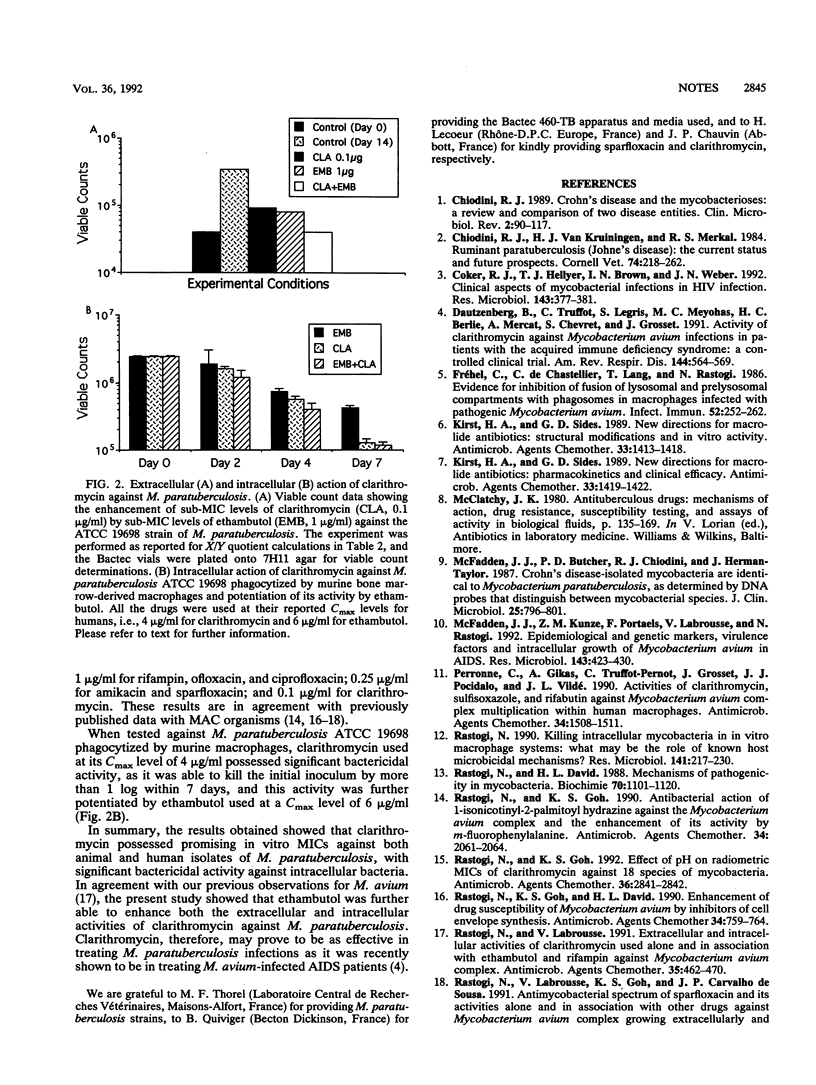
Radiometric MICs of clarithromycin, a new macrolide drug, were determined against five mycobactin-dependent strains of Mycobacterium paratuberculosis (including two Crohn's disease clinical isolates) and compared with those of other drugs which included rifampin, ethambutol, amikacin, ofloxacin, ciprofloxacin, and sparfloxacin. Among the drugs screened, clarithromycin was the drug for which MICs were lowest against the five strains tested. As MICs were significantly below the reported Cmax levels (about 4 micrograms/ml), the intracellular activity of clarithromycin against the type strain of M. paratuberculosis maintained in cultured macrophages was screened. Clarithromycin was able to kill the initial inoculum by more than 1 log within 7 days, and this activity was further potentiated by ethambutol. Extracellular drug combination screened by using sublethal concentrations of the drugs showed that ethambutol was able to enhance clarithromycin activity in three out of four M. paratuberculosis strains instead of only one out of four strains (or none in the case of ofloxacin) when associated with other drugs. These results suggest that clarithromycin may be fruitful to treat human disease in which M. paratuberculosis may be etiologically involved.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiodini R. J. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin Microbiol Rev. 1989 Jan;2(1):90–117. doi: 10.1128/cmr.2.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984 Jul;74(3):218–262. [PubMed] [Google Scholar]
- Coker R. J., Hellyer T. J., Brown I. N., Weber J. N. Clinical aspects of mycobacterial infections in HIV infection. Res Microbiol. 1992 May;143(4):377–381. doi: 10.1016/0923-2508(92)90049-t. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: pharmacokinetics and clinical efficacy. Antimicrob Agents Chemother. 1989 Sep;33(9):1419–1422. doi: 10.1128/aac.33.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: structural modifications and in vitro activity. Antimicrob Agents Chemother. 1989 Sep;33(9):1413–1418. doi: 10.1128/aac.33.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Kunze Z. M., Portaels F., Labrousse V., Rastogi N. Epidemiological and genetic markers, virulence factors and intracellular growth of Mycobacterium avium in AIDS. Res Microbiol. 1992 May;143(4):423-30, discussion 430-6. doi: 10.1016/0923-2508(92)90057-u. [DOI] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Pocidalo J. J., Vilde J. L. Activities of clarithromycin, sulfisoxazole, and rifabutin against Mycobacterium avium complex multiplication within human macrophages. Antimicrob Agents Chemother. 1990 Aug;34(8):1508–1511. doi: 10.1128/aac.34.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S. Action of 1-isonicotinyl-2-palmitoyl hydrazine against the Mycobacterium avium complex and enhancement of its activity by m-fluorophenylalanine. Antimicrob Agents Chemother. 1990 Nov;34(11):2061–2064. doi: 10.1128/aac.34.11.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Enhancement of drug susceptibility of Mycobacterium avium by inhibitors of cell envelope synthesis. Antimicrob Agents Chemother. 1990 May;34(5):759–764. doi: 10.1128/aac.34.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S. Effect of pH on radiometric MICs of clarithromycin against 18 species of mycobacteria. Antimicrob Agents Chemother. 1992 Dec;36(12):2841–2842. doi: 10.1128/aac.36.12.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N. Killing intracellular mycobacteria in in vitro macrophage systems: what may be the role of known host microbicidal mechanisms? Res Microbiol. 1990 Feb;141(2):217–230. doi: 10.1016/0923-2508(90)90034-n. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V. Extracellular and intracellular activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Mar;35(3):462–470. doi: 10.1128/aac.35.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxegaard F., Baess I. Relationship between Mycobacterium avium, Mycobacterium paratuberculosis and "wood pigeon mycobacteria". Determinations by DNA-DNA hybridization. APMIS. 1988 Jan;96(1):37–42. [PubMed] [Google Scholar]
- Schneebaum C. W., Novick D. M., Chabon A. B., Strutynsky N., Yancovitz S. R., Freund S. Terminal ileitis associated with Mycobacterium avium-intracellulare infection in a homosexual man with acquired immune deficiency syndrome. Gastroenterology. 1987 May;92(5 Pt 1):1127–1132. doi: 10.1016/s0016-5085(87)91068-7. [DOI] [PubMed] [Google Scholar]
- Takayama K., Armstrong E. L., Kunugi K. A., Kilburn J. O. Inhibition by ethambutol of mycolic acid transfer into the cell wall of Mycobacterium smegmatis. Antimicrob Agents Chemother. 1979 Aug;16(2):240–242. doi: 10.1128/aac.16.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Kilburn J. O. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989 Sep;33(9):1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M. In vitro activity of antimicrobial agents against the Mycobacterium avium complex inside macrophages from HIV1-infected individuals: the link to clinical response to treatment? Res Microbiol. 1992 May;143(4):411–419. doi: 10.1016/0923-2508(92)90055-s. [DOI] [PubMed] [Google Scholar]
- Yoshimura H. H., Graham D. Y. Nucleic acid hybridization studies of mycobactin-dependent mycobacteria. J Clin Microbiol. 1988 Jul;26(7):1309–1312. doi: 10.1128/jcm.26.7.1309-1312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



