Abstract
The serine-threonine kinase Akt is a downstream target of phosphoinositide 3-kinase (PI 3-kinase); it is activated by the phosphoinositide 3-phosphate-dependent kinases PDK1 and PDK2. Certain mutated forms of Akt induce oncogenic transformation in chicken embryo fibroblast cultures and hemangiosarcomas in young chickens. This ability to transform cells depends on localization of Akt at the plasma membrane and on the kinase activity of Akt. A transdominant negative form of Akt interferes with oncogenic transformation induced by the p3k oncogene, which codes for an activated form of PI 3-kinase. Akt is therefore an essential mediator of p3k-induced oncogenicity.
Keywords: phosphoinositide 3-kinase/oncogenic transformation/protein kinase B
Retroviral oncogenes are altered copies of cellular genes that code for constituents of cellular signaling pathways. A recent example of a cellular signaling component turning up as an oncogene in a retrovirus is v-p3k, which was isolated from the genome of avian sarcoma virus 16. v-p3k codes for a protein that is closely homologous to the catalytic subunit p110α of the phosphoinositide 3-kinase (PI 3-kinase). It induces oncogenic transformation of chicken embryo fibroblasts (CEF) in culture and hemangiosarcomas in young chickens (1). PI 3-kinase lies at a nodal point of multiple cellular signal chains and hence is a participant in numerous cellular processes (2). It receives signals from receptor and nonreceptor tyrosine kinases via its regulatory subunit, p85, which activates the enzyme by translocating it to the plasma membrane (3). An alternative mechanism proceeds through Ras, which binds directly to p110α and activates its catalytic activity (4, 5). One of the downstream targets of PI 3-kinase is the serine-threonine kinase Akt. Akt was identified as the product of an oncogene in a lymphomagenic murine retrovirus, AKT 8 (6). Cellular Akt (c-Akt) is also referred to as protein kinase B (PKB) (9). Akt has a functionally important pleckstrin homology (PH) domain at the amino terminus. Activation of Akt by PI 3-kinase involves binding of phosphatidylinositol-3,4,5-triphosphate and phosphatidylinositol-3,4-biphosphate to the PH domain, which results in the translocation of Akt to the plasma membrane (7). Activation also includes phosphorylation of Akt on T308 and S473 by the PI-dependent kinase PDK1 and an unidentified kinase referred to as PDK2 (8–11). In this paper, we show that constitutively activated Akt can transform CEF in culture and induce hemangiosarcomas in the animal, as does the v-P3k protein. We analyze the molecular domains that determine oncogenicity of Akt, and show that Akt is essential for oncogenic transformation induced by v-p3k.
MATERIALS AND METHODS
Akt Constructs.
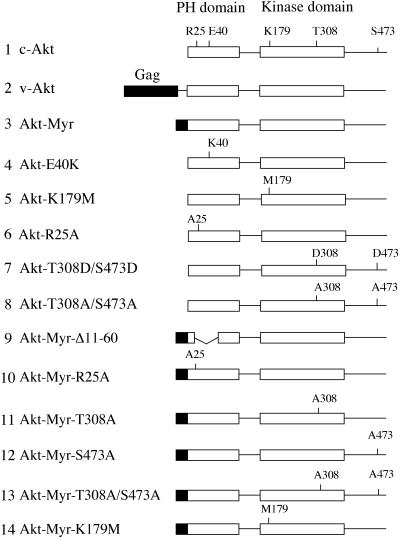
Wild-type akt and its mutants were described previously (12–14). Akt-Myr-S473A and Akt-Myr-T308A/S473A were generated by PCR using oligonucleotide primers and sequenced with an automated DNA sequencer (Applied Biosystems). All akt constructs were isolated from CMV5 or CMV6 expression vectors or from the MSVSRα retroviral vector and subcloned into the avian retroviral vector RCAS.Sfi, a modified version of RCAS (12, 15). RCAS.Sfi was generated by ligating the annealed oligonucleotides, 5′-CGGGCCATTACGGCCGATGATGATGACGACGGCCGCCTCGGCC-3′ and 5′-CGGGCCGAGGCGGCCGTCGTCATCATCATCGGCCGTAATGGCC-3′ into the ClaI cloning site of RCAS. RCAS.Sfi contains two incompatible SfiI sites to avoid vector self-ligation and to enable single-copy insert in a fixed orientation (16). An adaptor plasmid pBSFI was constructed by ligating two sets of annealed oligonucleotides as follows: 5′-GGCCATTACGGCCGCGGCCGCCTCTAGAGGATCCGATATCG-3′ annealed with 5′-AATTCGATATCGGATCCTCTAGAGGCGGCCGCGGCCGTAATGGCCAGCT-3′ and 5′-AATTCACGCGTAAGCTTATCGATGTCGACGGGCCCGGCCGCCTCGGCCGTAC-3′ annealed with 5′-GGCCGAGGCGGCCGGGCCCGTCGACATCGATAAGCTTACGCGTG-3′. The annealed oligonucleotides were subcloned into the KpnI, SacI-digested pBluescript SK (Stratagene). pBSFI contains multiple cloning sites flanked by two SfiI sites identical to the SfiI sites in RCAS.Sfi. Some of the akt sequences (c-akt, akt-E40K, and v-akt) were blunted with Klenow enzyme and directly subcloned into the original RCAS vector at the blunted ClaI site. RCAS coding for Env proteins of subgroup A was used, except for the interference assay described below, in which subgroup B constructs were used to express transdominant negative Akt. Fig. 1 is a schematic representation of the Akt constructs used in this study.
Figure 1.
Schematic structures of Akt constructs. Group-specific antigen (Gag) sequence in v-Akt or myristylation signals are highlighted as black boxes. Functionally important amino acid residues in the PH and kinase domains are noted. The HA epitope tag is on the amino terminus of constructs 1 and 4–8; on all others, it is on the carboxyl terminus.
Cell Culture, Transfection, and Induction of Tumors in Vivo.
Primary CEF cultures were prepared from White Leghorn embryos obtained from SPAFAS (Norwich, CT) as described (17). For focus assays, DNA was transfected into secondary CEF by using the dimethyl sulfoxide-polybrene method (1). For interference assays, secondary CEF cultures were transfected with subgroup B RCAS constructs. The cultures were passaged three times and then seeded on 35-mm plates at 6 × 105 cells. Focus assays with virus stocks were performed as described (18). After counting foci of transformed cells, the assay plates were stained with crystal violet and photographed. The oncogenicity of Akt in newly hatched chickens was tested by injecting 1 × 106 CEF transfected with individual Akt constructs into the wing web. These transfected CEF represent temporary sources of infectious RCAS that then efficiently spread to cells of the host and expresses the insert.
Western Blot and in Vitro Kinase Assays.
Cells were lysed in Nonidet P-40 lysis buffer (20 mM Tris⋅HCl, pH 7.5/150 mM NaCl/10% glycerol/1% Nonidet P-40/10 mM NaF/1 mM sodium pyrophosphate/1 mM sodium orthovanadate/1 mM EDTA) containing proteinase inhibitors, 1 mM AEBSF [4-(2-aminoethyl) benzenesulfonylfluoride⋅HCl], 100 KIU (kallikrein inhibitory units)/ml aprotinin, and 10 μg/ml leupeptin. For Western blots, lysates consisting of 40 μg of protein were separated by SDS/PAGE (10%) and transferred to Immobilon P membranes (Millipore). The membranes were then probed with anti-hemagglutinin (HA) mAb 12CA5 (generous gift from R. Lerner, The Scripps Research Institute) at a 1:1,000 dilution. After incubation with horseradish peroxidase-conjugated secondary antibody (Amersham), bound proteins were detected by incubation with a chemiluminescent substrate (Renaissance plus, Dupont/NEN) according to the manufacturer’s protocol.
Immune-complex kinase assays were performed as described (14). Lysates containing 400 μg of protein were incubated with the anti-HA antibody 12CA5 for 60 min at 4°C. Thirty microliters of a 50% suspension of protein G Sepharose (Amersham) was then added and incubated for 30 min at 4°C on a rotator. Immunoprecipitates were washed three times with Nonidet P-40 lysis buffer and once with Akt kinase buffer (20 mM Hepes, pH 7.4/10 mM MgCl2/10 mM MnCl2/1 mM DTT). The immune complex was then incubated at room temperature for 15 min in 20 μl of the kinase buffer with 0.1 μg/ml histone H2B, 2 μM ATP and 10 μCi (1 Ci = 37 GBq) of [γ-32P]ATP (3,000 Ci/mmol, NEN). Twenty microliters of 2× SDS/PAGE sample buffer was added to stop the reaction. The samples were loaded on 12.5% SDS/PAGE membranes and then transferred to poly(vinylidene difluoride) (PVDF) membranes. The membranes were exposed using Kodak X-Omat XAR5 film.
Immunofluorescence.
Cells grown on glass coverslips were washed with PBS (8.1 mM Na2HPO4/1.5 mM KH2PO4/137 mM NaCl/2.7 mM KCl) and fixed with 3% paraformaldehyde in PBS for 30 min at room temperature. After a second wash with PBS, the adherent cells were permeabilized with PBS containing 0.1% Triton X-100 (Sigma) for 30 min. A wash with PBS followed, and the coverslips were incubated with anti-HA monoclonal antibody (12CA5) at a dilution of 1:1000 for 60 min at room temperature in a humidified chamber, washed three times in PBS, and incubated with anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC; Sigma) for 30 min. The coverslips were again washed three times with PBS and once with distilled water and mounted on glass slides with Slowfade mounting medium (Molecular Probes)
RESULTS
Localization at the Plasma Membrane and Oncogenicity.
We determined the oncogenicity of several Akt constructs that differ in their affinity for the plasma membrane. These mutants of Akt were compared with c-Akt and to the Akt protein coded for by the murine lymphoma virus AKT8 (v-Akt) (Fig. 2; Table 1). The Akt constructs were fused to an HA epitope of influenza virus for immunological recognition, inserted into the avian retroviral expression vector RCAS, and transfected into CEFs (15). Five of the Akt constructs induced foci of transformed cells within 10 days of transfection: v-Akt, c-Akt with an amino-terminal myristylation signal (Akt-Myr), the two Akt-Myr constructs with mutations that inactivate the PH domain (Akt-Myr-Δ11–60 and Akt-Myr-R25A), and c-Akt with a point mutation that results in an increased affinity of the PH domain for phospholipids (Akt-E40K). Compared with these highly transforming constructs, the focus-forming ability of c-Akt was markedly decreased: although focus numbers were comparable to those induced by the active constructs, focus formation took 3 weeks, and the foci remained smaller and showed less multilayering. The mutant Akt-R25A was even less transforming than c-Akt; focus counts were reduced by a factor of about 20. Besides the oncogenic cellular phenotype, we observed in CEF cultures expressing transforming versions of Akt islands of highly vacuolated, adherent cells (Fig. 2). The vacuoles could be stained with Oil-Red-O, suggesting that activated Akt may not only induce neoplastic transformation but also may cause differentiation of CEF into adipocytes. The adipogenic effect of Akt has also been observed with mouse 3T3-LI cells (19, 20).
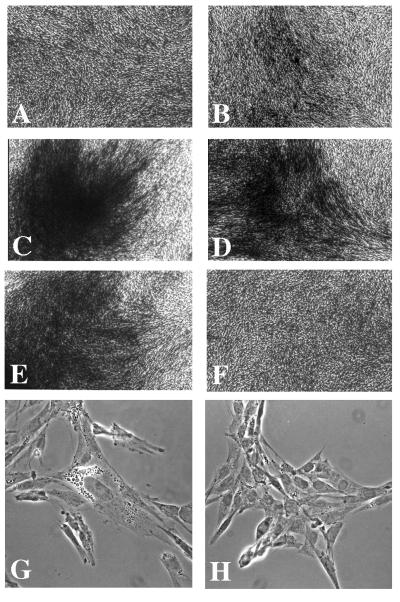
Figure 2.
Morphology of CEF transfected with Akt constructs. (A) RCAS vector control. (B) c-Akt. (C) v-Akt. (D) Akt-E40K. (E) Akt-Myr. (F) Akt-K179M. (G and H) Phase-contrast micrographs of CEFs transfected with Akt-Myr (G) or RCAS vector (H).
Table 1.
Transforming activity of Akt proteins
| Akt construct | Transformation of CEF | Tumorigenicity in chickens | Latent period, weeks |
|---|---|---|---|
| None (vector control) | − | 0/4 | |
| c-Akt | + | 1/7 | 4 |
| v-Akt | +++ | 3/7† | ≈1 |
| Akt-Myr | +++ | 7/8 | ≈1 |
| Akt-E40K | +++ | 7/7 | ≈1 |
| Akt-K179M | − | ND | |
| Akt-R25A | + | ND | |
| Akt-T308D/S473D | + | 0/4 | |
| Akt-T308A/S473A | − | ND | |
| Akt-Myr-Δ11-60 | +++ | 4/4 | ≈1 |
| Akt-Myr-R25A | +++ | 4/4 | ≈1 |
| Akt-Myr-T308A | − | 3/3 | 4 |
| Akt-Myr-S473A | ++ | 3/3 | 2-3 |
| Akt-Myr-T308A/S473A | − | 0/3 | |
| Akt-Myr-K179M | − | 0/2 |
Akt proteins were expressed in the RCAS vector. This vector produces infectious avian retroviral progeny, and this progeny virus was used in the transformation tests. The degree of transformation was rated by the size of transformed cell foci at 14 days past infection and the amount of multilayering in such foci. ND, not determined.
Tumors regressed.
In vivo Akt-E40K and the Akt-Myr constructs, Akt-Myr, Akt-Myr-Δ11–60, and Akt-Myr-R25A induced highly aggressive hemangiosarcomas within 1 week (Table 1). These tumors were histologically indistinguishable from the hemangiosarcomas induced by the PI 3-kinase homolog p3k. c-Akt also induced hemangiosarcomas, but the tumor incidence was lower, the latent period was 4 weeks, and the tumors grew distinctly slower. v-Akt caused very small tumors in about half of the birds within 1 week. However, these tumors regressed, presumably because of an immune response against the murine retroviral group-specific antigen sequences that are part of v-Akt.
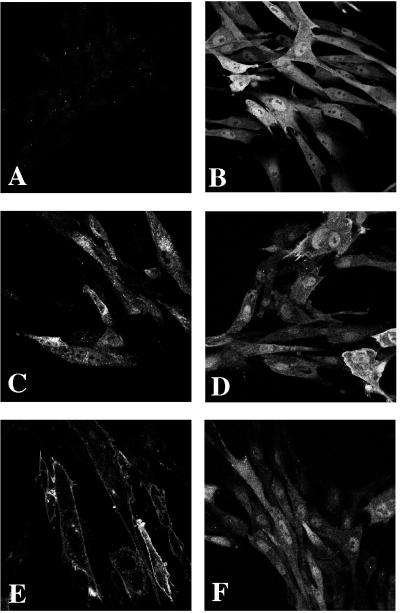
Akt proteins were visualized in transfected CEF by indirect immunofluorescence and confocal laser microscopy using anti-HA antibody (Fig. 3). Western blots carried out in parallel showed that all Akt constructs were expressed at similar levels in the transfected CEF (not shown). The microscopic examination revealed that c-Akt was distributed homogeneously throughout the nucleus and cytoplasm with no discernible affinity for the plasma membrane. The highly transforming constructs, v-Akt, Akt-Myr, Akt-Myr-Δ11–60, Akt-Myr-R25A, and Akt-E40K were concentrated at the plasma membrane. v-Akt and the Akt-Myr constructs also showed localization in a perinuclear area that appears to coincide with the endoplasmic reticulum. Akt-E40K produced a slight nuclear staining. The common denominator of the efficiently transforming Akt constructs is enhanced localization at the plasma membrane. This can be achieved by a PH domain with increased lipotropy (c-Akt-E40K) or by a myristylation signal that is either engineered in the amino-terminal region of the Akt-Myr constructs or is provided by viral group-specific antigen sequences that are fused to Akt in v-Akt. Membrane localization by means of a myristylation signal makes the PH domain functionally redundant for transformation: inactivating mutations in the PH domain, as in Akt-Myr-Δ11–60 and Akt-Myr-R25A have no effect on the high transforming ability of Akt-Myr constructs.
Figure 3.
Subcellular localization of the HA-tagged Akt proteins in CEF. (A) RCAS vector control. (B) c-Akt. (C) v-Akt. (D) Akt-E40K. (E) Akt-Myr. (F) Akt-K179M.
Kinase Activity and Oncogenicity.
The kinase activity of Akt is positively regulated by PDK1 and PDK2, which phosphorylate two sites in Akt, T308 and S473. We used mutants in these phosphorylation sites and in the kinase domain of Akt for tests of the role of kinase activity in the oncogenic transformation by comparing focus and tumor formation with kinase assays (Table 1 and Fig. 4). c-Akt and v-Akt served as reference constructs showing low and high transforming ability, respectively. The kinase-negative mutant Akt-K179M and Akt-Myr-K179M and mutants in which both of the PDK phosphorylation sites were replaced with alanines (Akt-T308A/S473A and Akt-Myr-T308A/S473A) failed to induce transformation in CEF cultures or tumors in the animal (Table 1). The Akt-Myr-T308A mutant with a substitution in a single PDK phosphorylation site but an active myristylation signal did not induce foci and produced only minute tumors after 4 weeks in the animal. Substitution of the other PDK site in Akt-Myr-S473A retained a reduced level of focus and tumor formation. A constitutively active Akt kinase was generated by replacing the PDK phosphorylation sites with aspartic acid residues (Akt-T308D/S473D). Despite elevated kinase level (see below) this mutant showed a low ability to transform CEF, characterized by a long latent period and small foci, similar to c-Akt. It did not induce tumors in vivo.
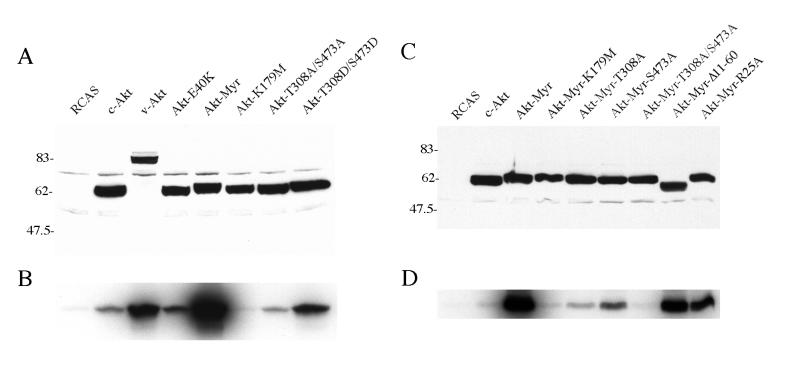
Figure 4.
Expression and in vitro kinase activity of Akt proteins. (A and C) Lysates of CEF stably transfected with HA-tagged Akt were probed with anti-HA antibody in Western blots. (B and D) In vitro immune complex kinase assay of the HA-tagged Akt constructs with histone H2B as substrate.
Immune-complex kinase assays were performed with histone H2B as substrate (Fig. 4). Control Western blots showed equal expression levels of the Akt constructs in the cells. v-Akt and Akt-T308D/S473D showed kinase activities that were significantly increased compared with that of c-Akt. Elevated kinase was also demonstrated with Akt-Myr and Akt-E40K. v-Akt, Akt-Myr, and Akt-E40K have a wild-type kinase domain and wild-type PDK phosphorylation sites, but share the ability to locate at the plasma membrane. The Akt-T308D/S473D mutant mimics constitutive phosphorylation at the positively regulatory PDK sites, which may explain its increased kinase activity. The Akt-T308A/S473A mutant retained a low kinase activity comparable to or lower than that of c-Akt, and Akt-K179M was kinase-negative. These data suggest that kinase activity is essential for oncogenic transformation by Akt as the kinase-defective mutants Akt-K179M, Akt-Myr-K179M, Akt-T308A/S473A, and Akt-Myr-T308A/S473A all failed to transform. However, an elevated kinase activity is not sufficient to cause effective transformation as shown by the Akt-T308D/S476D mutant, a poor transformer despite a high kinase level. This mutant does not show preferential localization at the plasma membrane, a prerequisite for high transforming activity. Selective membrane localization mediates the activation of the enzymatic function and of the ensuing oncogenic properties of Akt as is seen with v-Akt, Akt-E40K, and Akt-Myr.
Specific Interference by Akt with P3k-Induced Transformation.
Akt is a downstream target of PI 3-kinase and of the oncoprotein v-P3k. The close histological similarity of tumors induced by P3k and by Akt suggested that the oncogenic signal of P3k may travel through and require Akt. To test this possibility, the mutant Akt-T308A/S473A, which functions as a dominant negative form of Akt (21), was overexpressed in CEF by using an RCAS vector with a subgroup B envelope protein. Cells infected with this vector can still be superinfected with RCAS of envelope subgroup A and with other envelope A avian retroviruses. The superinfecting viruses were Prague strain of Rous sarcoma virus expressing the Src protein and RCAS-A expressing v-P3k. The transdominant negative Akt mutant induced a 25-fold greater resistance to challenge transformation by P3k; it was ineffective against Src (Table 2). Expression of both dominant negative Akt and v-P3k was confirmed by Western blot analysis (data not shown). This specific interference between dominant-negative Akt and P3k suggests that Akt is an essential component of the oncogenic signal originating in P3k.
Table 2.
Interference of dominant negative Akt with p3k-induced transformation*
| Transfected construct | Titer of challenge virus, focus-forming units/ml
|
||
|---|---|---|---|
| RCAS(A) | RCAS(A)-v-p3k | PR-RSV subgroup A | |
| RCAS(B) | 0 | 5 × 105 | 2 × 106 |
| RCAS(B)-Akt-T308A/S473A | 0 | 2 × 104 | 2 × 106 |
Data from a representative experiment.
DISCUSSION
Constitutively active forms of Akt induce oncogenic transformation of CEF and induce hemangiosarcomas in chickens. Dominant-negative Akt interferes with transformation by v-P3k, suggesting that the oncogenic signal from v-P3k is routed through Akt and that Akt is necessary for P3k oncogenesis. Preferential localization at the plasma membrane and elevated kinase activity both are essential requirements for the oncogenicity of Akt. A mutant that is a constitutively active kinase but lacks efficient direction to the plasma membrane (Akt-T308D/S473D) is a poor transformer, and conversely, membrane localization of a kinase-defective mutant (Akt-Myr-T308A/S473A or Akt-Myr-K179M) also fails to activate the oncogenic potential of Akt. Membrane localization appears to be the primary requirement and needs to be mutationally enhanced as in v-Akt, Akt-Myr, or Akt-E40K; it then mediates effective activation of the kinase function by PDK1 and PDK2 provided the PDK phosphorylation sites and the kinase domain are intact and functional (14).
Several oncogenes have been implicated in the induction of hemangiosarcomas in animals. Polyoma middle-T antigen induces hemangiosarcomas in chickens and in transgenic mice (22–25). Middle T is associated with the regulatory subunit p85 of PI 3-kinase and can activate PI 3-kinase and Akt (26, 27). Recent studies on mammary tumorigenesis in mice transgenic for polyoma middle T, expressed under control of the MMTV LTR, have shown that the oncogenic signal of middle T requires interaction with PI 3-kinase and with the Shc adaptor protein (28). A strain of avian erythroblastosis virus, harboring the v-erbB oncogene with an internal deletion in the cytoplasmic domain, was shown to induce hemangiosarcomas in chickens (29, 30). Such truncated epidermal growth factor receptors are also found in human brain tumors where they constitutively activate PI 3-kinase (31). Myristylated Fps, a nonreceptor tyrosine kinase, also induces hemangiosarcomas in transgenic mice, but its relationship to the PI-3 kinase pathway is not known (32, 33).
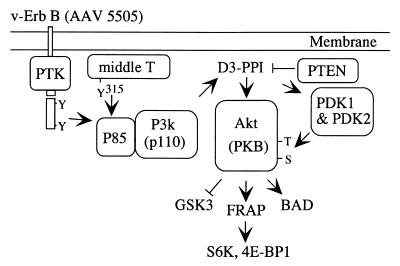
Akt has multiple ties to the cellular network of growth-controlling signals (Fig. 5). The upstream signal coming from PI 3-kinase is affected by the tumor suppressor Phosphatase-TENsin homolog PTEN/MMAC1, which dephosphorylates phosphatidylinositol-3,4,5-triphosphate (34). Mutations in PTEN have been detected in several human cancers (35, 36). These mutated PTEN proteins do not dephosphorylate phosphoinositides at the D3 position (D3-PPI), and an accumulation of D3-PPI may lead to the activation of Akt. Patients with the Bannayan Zoanna syndrome or with Cowden disease carry germ-line mutations in PTEN (37–40). These patients develop various types of tumors including hemangiomas. A downstream target of Akt is glycogen synthase kinase 3 (GSK-3), which is negatively regulated by Akt-dependent phosphorylation (41, 42). Reduced GSK-3 activity leads to increased levels of the growth stimulator β-catenin, which is a component of the Wnt-1-LEFI/TCF signaling pathway. Akt also activates FRAP (FK506 binding protein-rapamycin-associated protein; synonyms: mTOR, RAFT), a member of the ataxia telangiectasia family of PI 3-kinases (43). FRAP in turn phosphorylates and thereby activates the kinase for the ribosomal protein S6 (p70 S6 kinase) and the protein that binds to the eukaryotic initiation factor 4E, 4E-BP1 (44). These signals up-regulate the translation of certain mRNAs. The apoptosis-inducing protein BAD, another downstream target of Akt, is inactivated by Akt-dependent phosphorylation, thus enhancing cell survival (45, 46). All known Akt targets have the potential to affect cell growth. It will be important to identify those targets and signal chains that are essential for oncogenic transformation in cell culture and for the induction of hemangiosarcomas in the animal.
Figure 5.
Akt and cellular signaling. See text for details.
Acknowledgments
We thank Susan K. Burke for help with the manuscript. Oligonucleotides were synthesized by the Department of Molecular and Experimental Medicine Service laboratory supported by The Sam and Rose Stein Endowment Fund. This work was supported by U.S. Public Health Service Research Grant CA42564.
ABBREVIATIONS
- PI 3-kinase
phosphoinositide 3-kinase
- CEF
chicken embryo fibroblast(s)
- PKB
protein kinase B
- PH
pleckstrin homology
- PDK
phosphoinositide-dependent kinase
- Myr
myristylation signal
- CMV5
CMV6, MSVSRα, and RCAS, abbreviations for expression vectors
- v-p3k
retroviral p3k
- c-Akt
cellular Akt
References
- 1.Chang H W, Aoki M, Fruman D, Auger K R, Bellacosa A, Tsichlis P N, Cantley L C, Roberts T M, Vogt P K. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 2.Toker A, Cantley L C. Nature (London) 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 3.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M A, Williams L T. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Viciana P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Nature (London) 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa A, Testa J R, Staal S P, Tsichlis P N. Science. 1991;254:274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 7.Andjelkovic M, Alessi D R, Meier R, Fernandez A, Lamb N J, Frech M, Cron P, Cohen P, Lucocq J M, Hemmings B A. J Biol Chem. 1997;272:31515–24. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 8.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, et al. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 9.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 10.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 11.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G F, Holmes A B, Gaffney P R, Reese C B, McCormick F, Tempst P, et al. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 14.Bellacosa A, Chan T O, Ahmed N N, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 15.Hughes S H, Greenhouse J J, Petropoulos C J, Sutrave P. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miki T, Matsui T, Heidaran M A, Aaronson S A. Gene. 1989;83:137–146. doi: 10.1016/0378-1119(89)90411-3. [DOI] [PubMed] [Google Scholar]
- 17.Vogt P K. In: Fundamental Techniques in Virology. Habel K, Salzmann N P, editors. New York: Academic; 1969. pp. 198–211. [Google Scholar]
- 18.Bos T J, Monteclaro F S, Mitsunobu F, Ball A R, Jr, Chang C H, Nishimura T, Vogt P K. Genes Dev. 1990;4:1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- 19.Kohn A D, Summers S A, Birnbaum M J, Roth R A. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 20.Magun R, Burgering B M, Coffer P J, Pardasani D, Lin Y, Chabot J, Sorisky A. Endocrinology. 1996;137:3590–3593. doi: 10.1210/endo.137.8.8754791. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, et al. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautch V L, Toda S, Hassell J A, Hanahan D. IARC Sci Publ. 1989;96:255–266. [PubMed] [Google Scholar]
- 23.Kornbluth S, Cross F R, Harbison M, Hanafusa H. Mol Cell Biol. 1986;6:1545–1551. doi: 10.1128/mcb.6.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiestler O D, Aguzzi A, Williams R L, Wagner E F, Boulter C A, Kleihues P. IARC Sci Publ. 1989;96:267–274. [PubMed] [Google Scholar]
- 25.Williams R L, Risau W, Zerwes H G, Drexler H, Aguzzi A, Wagner E F. Cell. 1989;57:1053–1063. doi: 10.1016/0092-8674(89)90343-7. [DOI] [PubMed] [Google Scholar]
- 26.Dahl J, Jurczak A, Cheng L A, Baker D C, Benjamin T L. J Virol. 1998;72:3221–3226. doi: 10.1128/jvi.72.4.3221-3226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meili R, Cron P, Hemmings B A, Ballmer-Hofer K. Oncogene. 1998;16:903–907. doi: 10.1038/sj.onc.1201605. [DOI] [PubMed] [Google Scholar]
- 28.Webster M A, Hutchinson J N, Rauh M J, Muthuswamy S K, Anton M, Tortorice C G, Cardiff R D, Graham F L, Hassell J A, Muller W J. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson H L, Tracy S E, Nair N, Taglienti-Sian C, Gamett D C. Oncogene. 1992;7:2025–2030. [PubMed] [Google Scholar]
- 30.Tracy S E, Woda B A, Robinson H L. J Virol. 1985;54:304–310. doi: 10.1128/jvi.54.2.304-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moscatello D K, Holgado-Madruga M, Emlet D R, Montgomery R B, Wong A J. J Biol Chem. 1998;273:200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- 32.Greer P, Haigh J, Mbamalu G, Khoo W, Bernstein A, Pawson T. Mol Cell Biol. 1994;14:6755–6763. doi: 10.1128/mcb.14.10.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee S P, Mock D, Greer P, Maltby V, Rossant J, Bernstein A, Pawson T. Mol Cell Biol. 1989;9:5491–5499. doi: 10.1128/mcb.9.12.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maehama T, Dixon J E. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 36.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hatier T, Davis T, et al. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 37.Marsh D J, Dahia P L, Zheng Z, Liaw D, Parsons R, Gorlin R J, Eng C. Nat Genet. 1997;16:333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- 38.Arch E M, Goodman B K, Van Wesep R A, Liaw D, Clarke K, Parsons R, McKusick V A, Geraghty M T. Am J Med Genet. 1997;71:489–493. [PubMed] [Google Scholar]
- 39.Nelen M R, van Staveren W C, Peeters E A, Hassel M B, Gorlin R J, Hamm H, Lindboe C F, Fryns J P, Sijmons R H, Woods D G, et al. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- 40.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 41.Cross D A, Alessi D R, Cohen P, Andjelkovich M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 42.Shaw M, Cohen P, Alessi D R. FEBS Lett. 1997;416:307–311. doi: 10.1016/s0014-5793(97)01235-0. [DOI] [PubMed] [Google Scholar]
- 43.Scott P H, Brunn G J, Kohn A D, Roth R A, Lawrence J C., Jr Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence J C, Jr, Abraham R T. Trends Biochem Sci. 1997;22:345–349. doi: 10.1016/s0968-0004(97)01101-8. [DOI] [PubMed] [Google Scholar]
- 45.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 46.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]