Abstract
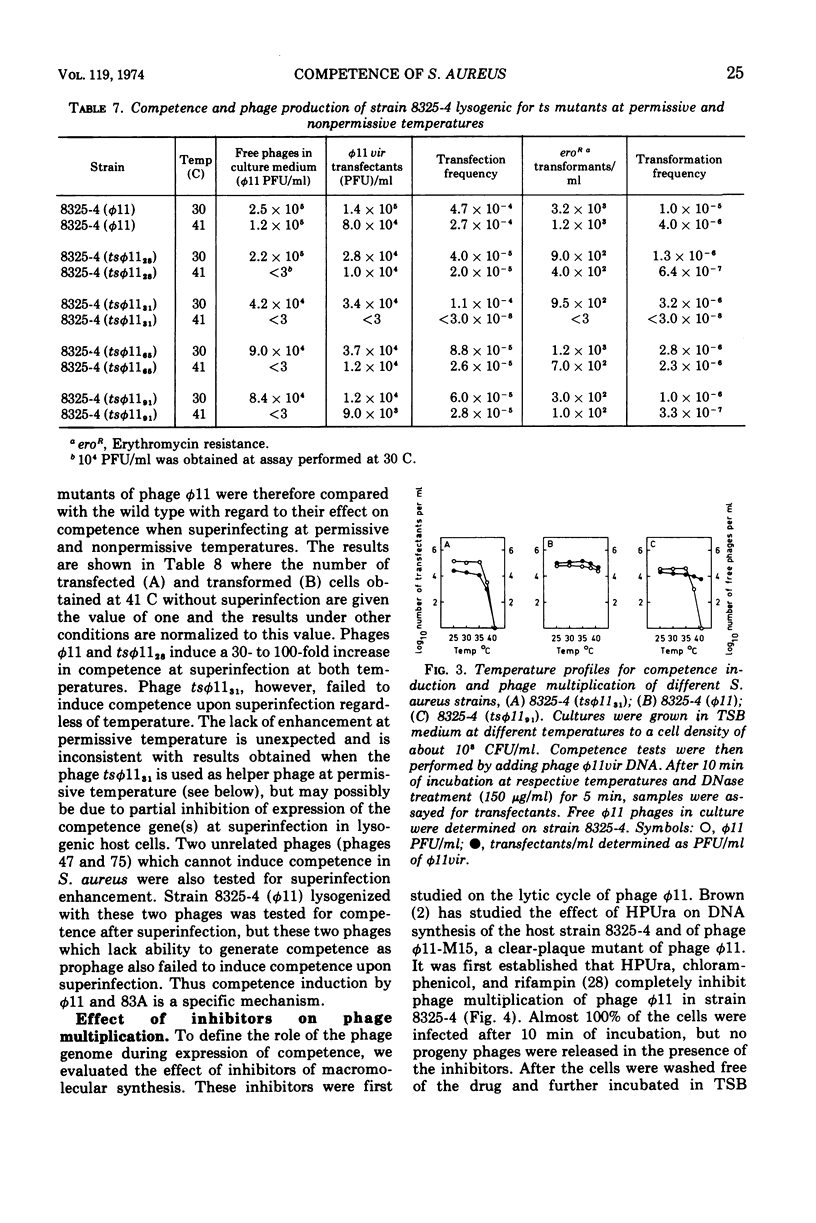
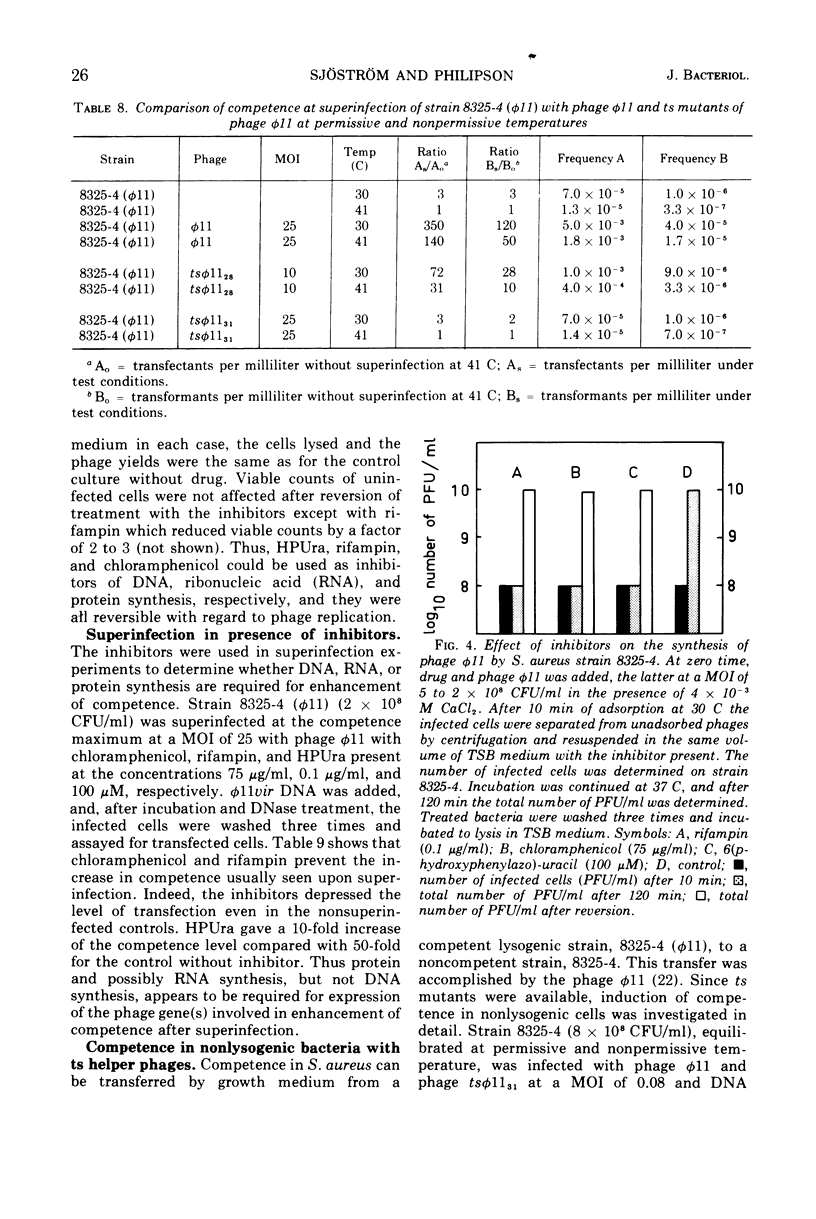
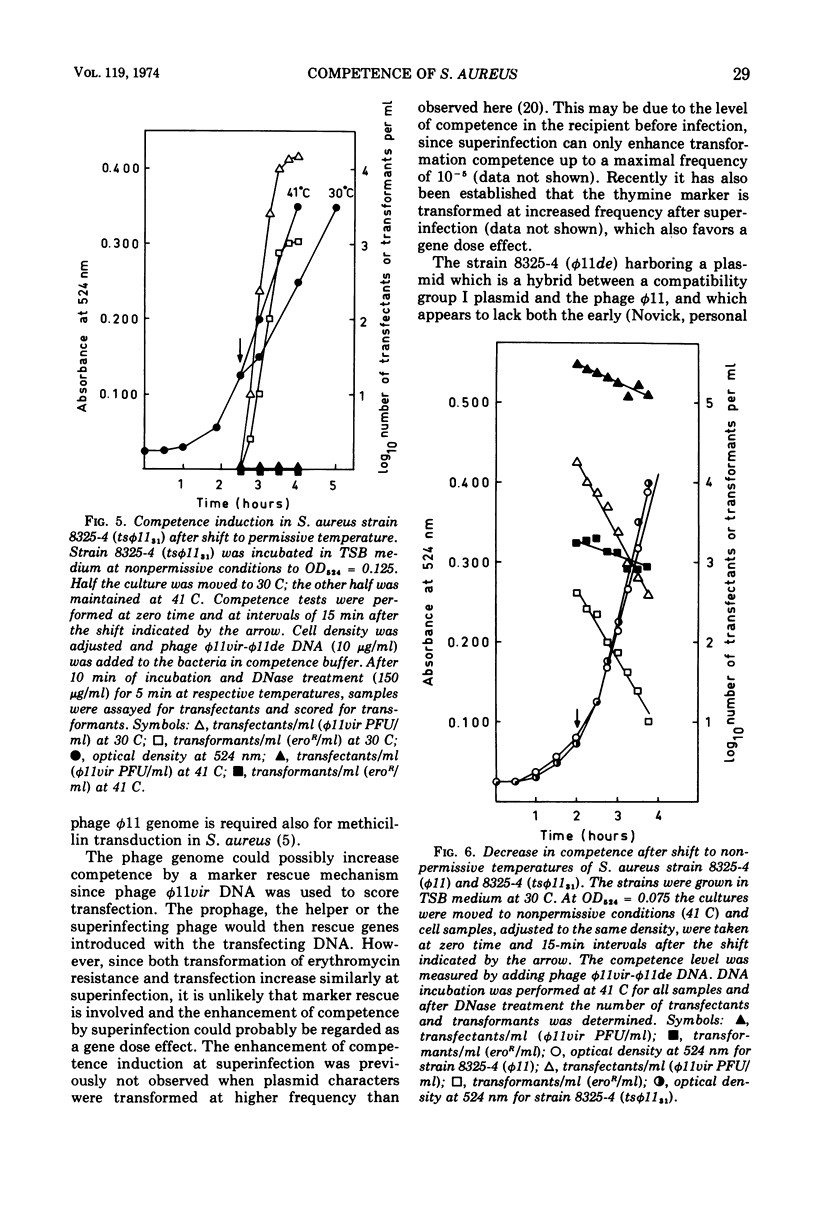
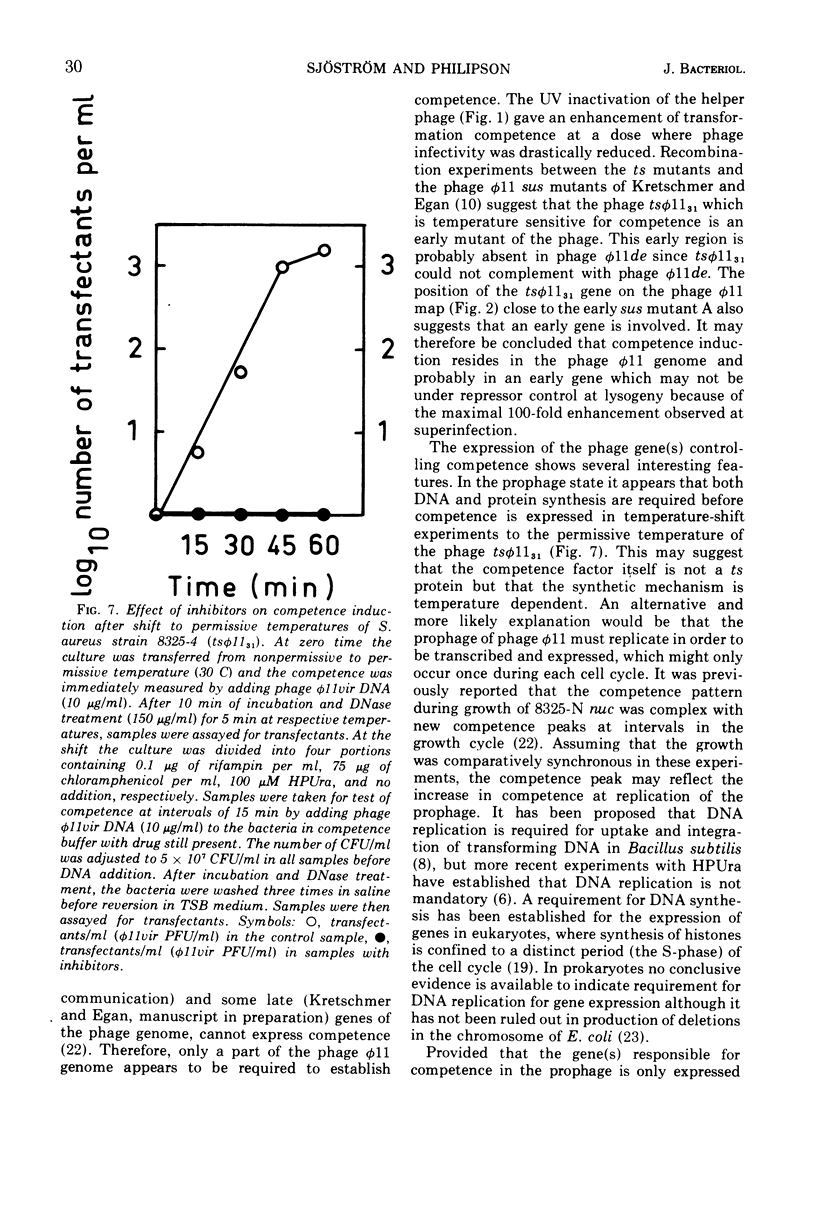
Both phage ø11 and 83A, when present as prophage or when used as helper phage, induce competence for transfection and transformation to the same level in Staphylococcus aureus, strain 8325-4. Cells lysogenized with certain temperature-sensitive (ts) mutants of phage ø11 show competence at the nonpermissive temperature (41 C) without production of infectious phages. Phage ø11ts allele 31 can neither as a prophage nor as a helper phage develop competence under nonpermissive conditions. This mutant appears, therefore, to be mutated in the region of the phage genome controlling competence. The competence level for both transfection and transformation is increased by superinfecting strain 8325-4 (ø11) or 8325-4 (83A) at high multiplicities with phage ø11 with some of its mutants or with phage 83A. This superinfection enhancement appears to require protein synthesis but not deoxyribonucleic acid synthesis as judged from studies with inhibitors of macromolecular synthesis. Besides the phage particle, no extracellular or cell-bound factors so far detected can induce competence. The phage-induced product conferring competence is rapidly synthesized by strain 8325-4 (tsø1131) after shift to permissive conditions, but requires deoxyribonucleic acid and protein synthesis to be expressed. Recombination between the sus mutants of phage ø11 of Kretschmer and Egan and tsø1131 indicate that competence is controlled by an early gene in the lytic cycle which may be expressed also in lysogenic cells. The phage product inducing competence appears to have a half-life of 10 to 15 min in the conditional lethal mutant at shift to nonpermissive temperature. Ultraviolet inactivation of phage ø11 infectivity occurs more rapidly than inactivation of competence induction. In fact, the number of transformants is increased at low doses of irradiation. Competence induction is, however, decreased at high does of ultraviolet irradiation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. C. 6(p-Hydroxyphenylazo)-uracil: a reversible, selective inhibitor of the replication of deoxyribonucleic acid of staphylococcal bacteriophage P11-M15. J Virol. 1971 Nov;8(5):759–765. doi: 10.1128/jvi.8.5.759-765.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak M., Dedonder R. Production d'un "facteur de compétence" soluble par Bacillus subtilis Marburg ind 168. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 24;260(21):5638–5641. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):803–811. doi: 10.1128/jb.116.2.803-811.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: nonrequirement of deoxyribonucleic acid replication for uptake and integration of transforming deoxyribonucleic acid. J Bacteriol. 1973 Mar;113(3):1512–1514. doi: 10.1128/jb.113.3.1512-1514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. J., Braun W. Apparent dependence of transformation on the stage of deoxyribonucleic acid replication of recipient cells. Bacteriol Rev. 1968 Dec;32(4 Pt 1):291–296. [PMC free article] [PubMed] [Google Scholar]
- Higa A., Mandel M. Factors influencing competence of Escherichia coli for lambda-phage deoxyribonucleic acid infection. Jpn J Microbiol. 1972 Jul;16(4):251–257. doi: 10.1111/j.1348-0421.1972.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Kretschmer P. J., Egan J. B. Isolation of a suppressor host bacterium in Staphylococcus aureus. J Bacteriol. 1973 Oct;116(1):84–87. doi: 10.1128/jb.116.1.84-87.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg M., Sjöström J. E., Johansson T. Transformation of chromosomal and plasmid characters in Staphylococcus aureus. J Bacteriol. 1972 Feb;109(2):844–847. doi: 10.1128/jb.109.2.844-847.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mandel M. Infectivity of phage P2 DNA in presence of helper phage. Mol Gen Genet. 1967;99(1):88–96. doi: 10.1007/BF00306461. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Oishi M., Cosloy S. D. The genetic and biochemical basis of the transformability of Escherichia coli K12. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1568–1572. doi: 10.1016/0006-291x(72)90520-7. [DOI] [PubMed] [Google Scholar]
- PAKULA R., WALCZAK W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963 Apr;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M. Relationship between competence for transfection and for transformation. J Virol. 1968 Jun;2(6):587–593. doi: 10.1128/jvi.2.6.587-593.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin L., Sjöström J. E., Lindberg M., Philipson L. Factors affecting competence for transformation in Staphylococcus aureus. J Bacteriol. 1974 Apr;118(1):155–164. doi: 10.1128/jb.118.1.155-164.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Competence for transfection in Staphylococcus aureus. J Bacteriol. 1973 Feb;113(2):576–585. doi: 10.1128/jb.113.2.576-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Lindberg M., Philipson L. Transfection of Staphylococcus aureus with bacteriophage deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):285–291. doi: 10.1128/jb.109.1.285-291.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Horn V., Yanofsky C. On the production of deletions in the chromosome of Escherichia coli. J Mol Biol. 1970 Oct 14;53(1):49–67. doi: 10.1016/0022-2836(70)90045-8. [DOI] [PubMed] [Google Scholar]
- THOMAS R. Recherches sur la cinétique des transformations bactériennes. Biochim Biophys Acta. 1955 Dec;18(4):467–481. doi: 10.1016/0006-3002(55)90137-2. [DOI] [PubMed] [Google Scholar]
- TOMASZ A., HOTCHKISS R. D. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964 Mar;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo A. Sensitivity of Escherichia coli to viral nucleic acid. V. Competence of calcium-treated cells. J Biochem. 1972 Oct;72(4):973–979. doi: 10.1093/oxfordjournals.jbchem.a129988. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Staehelin M. Action of rifamycin on RNA-polymerase from sensitive and resistant bacteria. Biochem Biophys Res Commun. 1968 Jul 26;32(2):284–288. doi: 10.1016/0006-291x(68)90382-3. [DOI] [PubMed] [Google Scholar]


