Abstract
Our preliminary family studies have suggested that some female first-degree relatives of women with polycystic ovary syndrome (PCOS) have hyperandrogenemia per se. It was our hypothesis that this may be a genetic trait and thus could represent a phenotype suitable for linkage analysis. To investigate this hypothesis, we examined 115 sisters of 80 probands with PCOS from unrelated families. PCOS was diagnosed by the combination of elevated serum androgen levels and ≤6 menses per year with the exclusion of secondary causes. The sisters were compared with 70 healthy age- and weight-comparable control women with regular menses, no clinical evidence of hyperandrogenemia, and normal glucose tolerance. Twenty-two percent of the sisters fulfilled diagnostic criteria for PCOS. In addition, 24% of the sisters had hyperandrogenemia and regular menstrual cycles. Circulating testosterone (T) and nonsex hormone-binding globulin-bound testosterone (uT) levels in both of these groups of sisters were significantly increased compared with unaffected sisters and control women (P < 0.0001 for both T and uT). Probands, sisters with PCOS, and hyperandrogenemic sisters had elevated serum luteinizing hormone levels compared with control women. We conclude that there is familial aggregation of hyperandrogenemia (with or without oligomenorrhea) in PCOS kindreds. In affected sisters, only one-half have oligomenorrhea and hyperandrogenemia characteristic of PCOS, whereas the remaining one-half have hyperandrogenemia per se. This familial aggregation of hyperandrogenemia in PCOS kindreds suggests that it is a genetic trait. We propose that hyperandrogenemia be used to assign affected status in linkage studies designed to identify PCOS genes.
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies among women of reproductive age (1, 2). It has been proposed that PCOS has a genetic basis because a high number of female relatives are affected (1–5). These studies have been constrained by a failure to systematically examine PCOS women and their female relatives compared with concurrently studied control women to define precisely biochemical phenotypes. Indeed, most studies have evaluated relatives by questionnaires (3, 4), and this may have resulted in a miscalculation of the number of affected subjects. Other studies have used ovarian morphology (polycystic ovaries) rather than the endocrine abnormalities of the syndrome (hyperandrogenemia and chronic anovulation), to assign affected status (6, 7). The polycystic ovary morphology, however, can be present in ovulatory women with hyperandrogenemia (8) and in women who are endocrinologically normal (9).
Our preliminary family studies have suggested that some female first-degree relatives of PCOS probands have hyperandrogenemia per se (1, ‖). It is our hypothesis that the endocrine abnormalities of PCOS have a genetic basis and that hyperandrogenemia may be an additional phenotype (1, 10, ‖). In preparation for linkage studies to identify PCOS genes, we sought to determine the reproductive endocrine phenotypes of female first-degree relatives in PCOS kindreds. Because the phenotype may be expressed only during the reproductive years (10), we examined the sisters of PCOS probands to accomplish this. We report familial clustering of reproductive endocrine biochemical abnormalities in PCOS kindreds, suggesting that these traits have a genetic basis.
METHODS
Subjects.
The study population consisted of 115 sisters of 80 unrelated women with PCOS. Age-, ethnicity-, and weight-comparable unrelated control women (n = 70) were recruited who did not have a history of hypertension or of diabetes mellitus, either personally or in their first-degree relatives. All PCOS probands and control women were in good health and, for at least 1 month before each study, were not taking any medication known to affect sex hormone metabolism (except for contraceptive steroids, which were stopped for at least 3 months before the study). The ethnicity of the control group was 87% non-Hispanic white, 10% Hispanic, and 3% African American. The control women had regular menses every 27–35 days and no hirsutism (Ferriman-Gallwey score <8; ref. 11) or acne was present during physical examination. All control women had normal glucose tolerance on a 75-g 2-hr oral glucose-tolerance test using World Health Organization criteria (12). The diagnosis of PCOS was made using as criteria an elevation of circulating androgen levels, either testosterone (T) or nonsex hormone-binding globulin-bound testosterone (uT) associated with chronic oligomenorrhea (≤6 menses per year) or amenorrhea (13, 14). Women with nonclassical 21-hydroxylase deficiency, hyperprolactinemia, and androgen-secreting tumors were excluded by using appropriate tests (14).
Families studied consisted of a proband who fulfilled our diagnostic criteria for PCOS and at least one sister. Probands were recruited from the clinical practices of the authors, referrals to the study, and advertisements in local papers. The majority of the probands (63/80) were from the central Pennsylvania area. Seventy-five probands were non-Hispanic white, four were Hispanic, and one was African American. All available sisters were studied. The kindreds included 134 sisters, 115 of whom participated in the study. A total of 19 sisters from 13 families were unable or unwilling to be studied. Two of these unstudied sisters were currently using contraceptive steroids and one was pregnant. Sisters who were studied ranged in age from 11 to 54 years. Sisters were not required to stop hormonal medications before their visit. Three families had 4 sisters, 4 had 3 sisters, 18 had 2 sisters, and 55 had only 1 sister studied. The study was approved by the Institutional Review Boards of the Hershey Medical Center and the University of Pennsylvania Medical Center, and all of the women gave written informed consent.
All control women and probands were examined by one of the study investigators. Because of their distance from our study sites, 39 sisters had blood drawn at an outside site. These women were not examined by a study investigator. Height, weight, and blood pressure were obtained, and body mass index (BMI, kg/m2) was determined in all subjects. A single fasting blood sample was obtained between 8:00 and 10:00 a.m.
Assays.
Assays for T and dehydroepiandrosterone sulfate (DHEAS) were performed by using Diagnostic Products (Los Angeles) Coat-A-Count kits; the interassay coefficients of variation (CVs) were 8% and 5%, respectively (14). Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) assays were performed by using Diagnostic Product double antibody kits; the interassay CVs were 6% and 9%, respectively (14). Unbound testosterone was measured by a modification of the procedure of Tremblay and Dube (14, 15); the interassay CV was 7%. Unbound T could not be assayed in 2 subjects (1 proband and 1 sister) because of gross lipemia. Gonadotropin levels were measured on 70 probands, 101 sisters, and 67 control women. An insufficient blood sample for gonadotropin assays was obtained in 27 women (10 PCOS probands, 14 sisters, and 3 control women).
Phenotypes.
Unaffected status (normal) was defined as sisters with regular menstrual cycles every 27–35 days and normal circulating androgen levels. The PCOS phenotype was the combination of an elevated circulating T level (either total T or uT 2 SD above the control mean value) with ≤6 menses per year (13). Sisters were considered to be hyperandrogenemic alone if they had levels of T or uT that were greater than 2 SD above the control mean values and they had regular menstrual cycles. We assigned unknown diagnostic status to 36 sisters for the following reasons. Reproductive-age women were classified as unknown if they were on oral (n = 17) or depot contraceptive agents, i.e., levonorgestrel implants (Norplant; Wyeth Ayerst Laboratories, Marietta, PA) or medroxyprogesterone acetate depot injection (Depo-Provera; Pharmacia & Upjohn; n = 2) (16). Unknown status also was assigned to two sisters on medication that could potentially affect sex hormone metabolism [i.e., metformin (17) or verapamil (18)]. Unknown status was assigned to postmenopausal women (n = 5). Unknown status also was assigned if we were unable to obtain a blood sample (n = 1), if there was a history of ≤6 menses per year and no hyperandrogenemia (n = 6), or if there was an isolated DHEAS elevation.
Normative Data.
We initially recruited 43 women to serve as controls for defining the normal range for serum androgen levels. Thirty-six were non-Hispanic white and seven were Hispanic. The age range of these women was 18–40 years old. Control mean values ± SD (n = 43) for T were 32.0 ± 13.0 ng/dl, for uT were 7.0 ± 4.0 ng/dl, and for DHEAS were 1,451 ± 616 ng/ml (× 0.03467 = nmol/liter). We defined our normal range for T and uT levels as greater than 2 SD (95% confidence intervals) above the control mean value: T > 58 ng/dl, uT > 15 ng/dl, and DHEAS > 2,683 ng/ml.
We continued to recruit control women to ensure the ongoing validity of the normative database. To date, we have examined a total of 70 control women. We have included those who had a T or a uT measurement above our normal range (10/70) to reflect the prevalence of hyperandrogenemia in the control population. The distribution of androgen values in the control population was as follows: for total T, the 10th percentile was 20 ng/dl, the 50th percentile was 33 ng/dl, and the 90th percentile was 62 ng/dl. For uT, the 10th percentile was 3 ng/dl, the 50th percentile was 8 ng/dl, and the 90th percentile was 17 ng/dl.
Data Analysis.
Data were analyzed by using ANOVA with Scheffe’s post hoc procedure. We used all of the control women studied (n = 70) for our control group in these analyses. Gonadotropin data were log-transformed for ANOVA to achieve homogeneity of variance. We did not include the sisters of unknown status in the data anaysis. Six unaffected sisters were deleted from the analysis of the gonadotropin data who appeared to have a midcycle surge on the day of their visit (both LH and FSH >20 milliunits/ml). Data were analyzed by using statview 4.5 for the Macintosh (Abacus Concepts, Berkeley, CA). A P ≤ 0.05 was considered to be significant. All data are presented as the untransformed mean ± SD.
RESULTS
The prevalence of phenotypes among sisters of PCOS women is summarized in Table 1. In calculating the percent of sisters affected, sisters of unknown status (n = 36) were excluded from the denominator. This left 79 sisters in the denominator. Overall, 22% of sisters of PCOS probands fulfilled our criteria for diagnosis of PCOS. An additional 24% of sisters had elevated circulating androgen levels (either T or uT) and regular menstrual cycles (HA sisters). The most common phenotype found among sisters was hyperandrogenemia with or without oligomenorrhea (i.e., 22% + 24% = 46%). In comparison, 14% (10/70) of control women had a T and/or a uT level above the normal range. This was a significantly different distribution of hyperandrogenemic phenotypes in sisters compared with control women (χ2 = 21.0; P < 0.0001). Sisters of PCOS women had a ≈3-fold greater risk of being hyperandrogenemic compared with control women (46%/14% = 3.3). The majority of sisters reporting ≤6 menses per year (n = 28) had either elevated androgen levels (n = 17) or were on hormonal suppression (n = 5). Unexplained menstrual irregularity was found in only six sisters. Fifty-four percent of sisters (n = 43) had regular menses and androgen levels within the control range and were designated unaffected (UA).
Table 1.
Definition and prevalence of phenotypes in sisters
| Phenotype of sister | No. affected (total, n = 115) | % affected | Summary |
|---|---|---|---|
| PCOS | 17 | 22 | Premenopausal, ≤6 menses per yr, and elevated T and/or uT levels |
| Hyperandrogenemia alone | 19 | 24 | Premenopausal, menses 27-35 d, elevated T and/or uT levels |
| Unaffected | 43 | 54 | Premenopausal, menses 27-35 d, normal T, uT, and DHEAS levels |
| Unknown | 36 | — | Women on oral contraceptives or on confounding medications (n = 21), postmenopausal (n = 5), irregular menses with normal androgens (n = 6), isolated DHEAS elevation (n = 3), and unable to obtain blood (n = 1). |
Unknown sisters were excluded from the denominator when percentages were calculated; denominator = 83.
Table 2 summarizes the clinical features, excluding sisters with unknown status. The age range for the PCOS probands was 17–43 years old, for sisters was 11–53 years old, and for control women was 18–40 years old. By design, there were no significant differences in mean age or BMI among PCOS probands, sisters, and control women. However, when sisters were subdivided according to phenotype, differences in mean BMI and age became apparent. PCOS probands and sisters with PCOS had a significantly elevated mean BMIs compared with UA sisters. UA sisters had significantly lower mean BMIs compared with control women. PCOS probands and sisters with PCOS had fewer numbers of pregnancies and deliveries, whereas HA sisters had similar numbers of pregnancies and deliveries compared with UA sisters and control women (Table 2).
Table 2.
Clinical features
| Study group | n | Age, yrs | BMI, kg/m2 | Gravidity | Parity |
|---|---|---|---|---|---|
| PCOS probands | n = 80 | 29 ± 7 | 33.3 ± 8.2† | 0.6 ± 1.0‡ | 0.3 ± 0.7†‡ |
| PCOS sisters | n = 17 | 25 ± 7 | 33.6 ± 9.0† | 0.4 ± 0.7‡ | 0.2 ± 0.6‡ |
| HA sisters | n = 19 | 26 ± 7 | 28.8 ± 6.1 | 1.3 ± 1.4 | 0.9 ± 1.1 |
| UA sisters | n = 43 | 31 ± 10 | 26.0 ± 6.8‡ | 1.3 ± 1.4 | 1.1 ± 1.3 |
| Control subjects | n = 70 | 30 ± 7 | 31.7 ± 8.9 | 1.7 ± 1.9 | 1.2 ± 1.4 |
| ANOVA P-value | 0.04 | <0.0001 | <0.0001 | <0.0001 |
Values are given as mean ±SD. P values were derived from ANOVA with Scheffe’s post hoc test. Significant values are noted:
, vs. UA sisters;
, vs. control subjects.
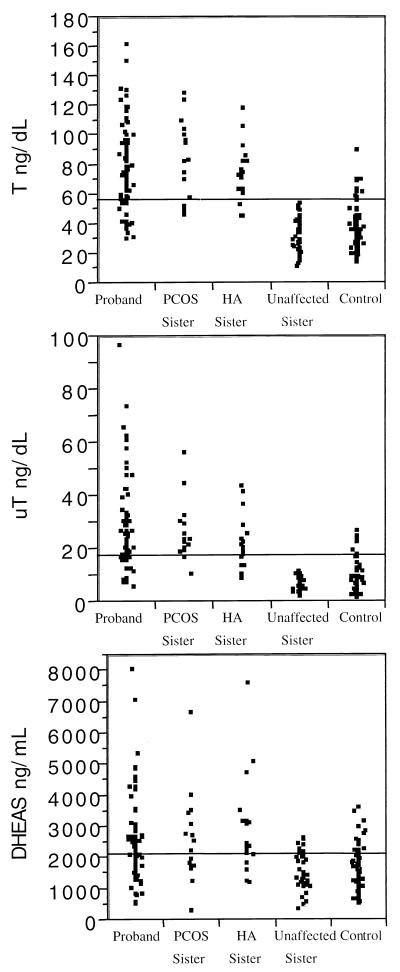
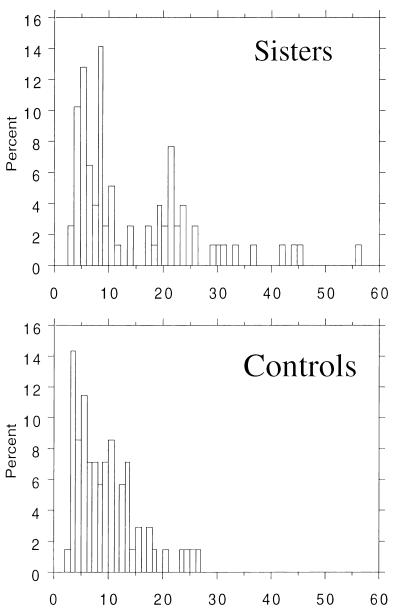
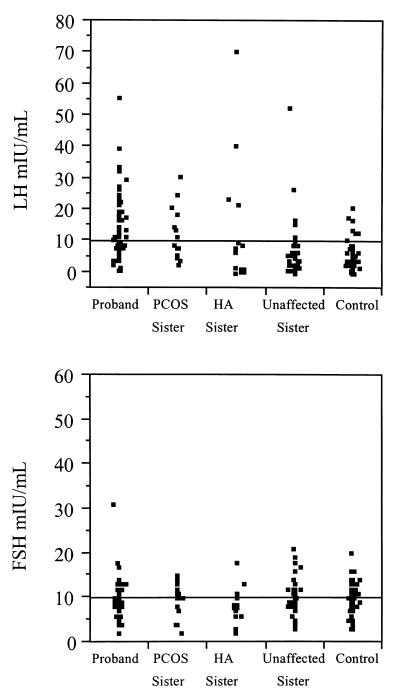
PCOS and HA sisters had androgen values similar to those of PCOS probands, and UA sisters had androgen values similar to control women (Fig. 1). The distribution of T and uT levels in the sisters appeared to be bimodal, whereas it was unimodal in the control women (Fig. 2). PCOS probands, sisters with PCOS, and HA sisters all had significantly elevated mean T and uT levels compared with both the UA sisters and control women (Table 3). DHEAS levels were significantly elevated in PCOS probands, sisters with PCOS, and HA sisters compared with control women and with UA sisters (Table 3). HA sisters had significantly increased LH levels compared with control women as well as to PCOS probands, sisters with PCOS, and UA sisters (Table 3). Two unrelated HA sisters had markedly elevated LH levels (135 and 138 milliunits/ml, respectively) that were confirmed on repeat assay, with normal FSH levels (2 and 3 milliunits/ml, respectively). A number of additional HA sisters had LH levels similar to PCOS probands and sisters with PCOS (Fig. 3). PCOS probands had significantly elevated LH levels compared with control women (Table 3). LH levels in all of the affected women overlapped with the levels in UA sisters and control women (Fig. 3). There were no significant differences in mean FSH levels.
Figure 1.
Distribution of serum androgen levels in control women (control), hyperandrogenemic sisters (HA Sister), unaffected sister, sisters with PCOS (PCOS Sister) and PCOS probands (Proband). A proband with a T value of 330 ng/dL and an HA sister with a T value of 260 ng/dl were excluded from the figure to better display the distribution of T levels.
Figure 2.
Frequency distribution of uT levels in sisters of PCOS probands (n = 78; Upper) and control women (n = 70; Lower). The distribution of the sisters appears to be bimodal (21).
Table 3.
Biochemical parameters in PCOS probands, sisters, and control women
| Study group | T, ng/dl | uT, ng/dl | DHEAS, ng/ml | LH, millunits/ml | FSH, millunits/ml |
|---|---|---|---|---|---|
| PCOS probands | 81 ± 40 (80)¶‖ | 30 ± 26 (79)† | 2,550 ± 1370 (80)¶‖ | 15 ± 10‖ (70) | 10 ± 4 (70) |
| PCOS sisters | 82 ± 27 (17)¶‖ | 26 ± 11 (16)¶‖ | 2,600 ± 1360 (17)‖ | 13 ± 8 (15)‖ | 10 ± 3 (15) |
| HA sisters | 86 ± 46 (19)¶‖ | 23 ± 10 (19)¶‖ | 2,990 ± 1520 (19)¶‖ | 34 ± 47 (14)†‡§¶‖ | 9 ± 4 (14) |
| UA sisters | 32 ± 12 (43) | 6 ± 2 (43) | 1,610 ± 720 (43) | 8 ± 9 (36) | 11 ± 4 (36) |
| Control subjects | 32 ± 12 (70) | 9 ± 6 (70) | 1,650 ± 730 (70) | 5 ± 4 (67) | 10 ± 4 (67) |
| ANOVA, P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.40 |
All values are given as mean ±SD (n). Significant values obtained with Scheffe’s post hoc test are noted:
, vs. PCOS probands;
, vs. PCOS sisters;
, vs. HA sisters;
, vs. UA sisters;
, vs. control subjects.
Figure 3.
Distribution of LH and FSH levels determined in a single blood sample in control women, hyperandrogenemic sisters (HA Sister), unaffected sisters, PCOS sisters (PCOS Sister) and PCOS probands (Probands). Two HA sisters with LH levels of 135 and 138 milliunits/ml, respectively, were excluded from the figure to better show the distribution of LH values.
DISCUSSION
We have found that there is familial aggregation of hyperandrogenemia in PCOS kindreds, with 46% of sisters affected. Only one-half of these sisters fulfilled diagnostic criteria for PCOS with chronic anovulation (≤6 menses per year) and hyperandrogenemia (13). The remaining affected sisters had hyperandrogenemia with regular menses. The elevation of DHEAS levels in the affected sisters suggests that there was an adrenal component to the hyperandrogenemia (19). There was also familial clustering of LH abnormalities in both groups of hyperandrogenemic sisters, those with PCOS, and those with regular menses, suggesting that this may be an additional feature of the affected phenotype. The familial aggregation of reproductive endocrine abnormalities suggests that these traits have a genetic basis (5).
The distribution of testosterone levels in the sisters appeared to be bimodal (20). Although the sample size was relatively small, this bimodality suggests that testosterone levels in PCOS families may be a monogenic trait controlled by two alleles at an autosomal locus (20). A possible candidate gene would be one involved in the regulation of both ovarian and adrenal steroidogenesis, as DHEAS levels were also increased (2, 19). LH levels were increased in affected sisters. This increase appeared to be a less consistent defect. However, because abnormalities in LH secretion can escape detection in a single blood sample, more detailed studies of pulsatile LH release will be necessary to determine the familial prevalence of this neuroendocrine defect (21). These abnormalities in LH release may be secondary rather than primary because adrenal hyperandrogenism was also present, consistent with an intrinsic defect in steroidogenesis. It remains possible that the LH abnormalities reflect a second genetic trait (5). Adrenal hyperandrogenism has been reported to be a familial trait in the absence of congenital adrenal hyperplasia in PCOS (22). A recent preliminary report from Azziz and Kahsar-Miller** also suggested that ≈50% of 30 sisters of PCOS women had reproductive endocrine abnormalities by history, physical examination, and/or androgen levels.
Our study confirms earlier reports that there is familial clustering of PCOS. These studies have focused primarily on the ovarian morphology as determined by ultrasound (2, 4, 6, 23). Polycystic ovaries, however, are not a specific marker for the endocrine syndrome of PCOS. Indeed, more than 20% of normal female volunteers with regular menstrual cycles have polycystic ovaries observable by ultrasound (9, 24). Studies in PCOS families (4, 5, 7, 23) have consistently found that an even higher percentage of sisters have polycystic ovaries. However, few of these studies have extended phenotyping to include endocrine parameters (6, 7). One such study found that only 43% of female relatives who had polycystic ovaries also had an elevated testosterone value (7). Another study (25) suggested that there were increased insulin levels among first-degree relatives, although some of the sisters were reported to have increased androgen levels with normal menses. A twin study showed no concordance in androgen levels among either monozygotic or dizygotic twins who had a high prevalence of polycystic ovaries as observed by ultrasound (6). All of these studies were constrained by the high background rate of polycystic ovaries in the normal population (9, 24).
Biochemical phenotyping offers several advantages over ovarian sonography. First, it can be accomplished with a single blood test assayed in a central laboratory. Second, the background rate of “abnormal” values in the control population can be standardized by carefully establishing normal criteria. Third, biochemical criteria are objective and are not subject to operator interpretation, as is the case with ovarian sonography (9, 24). We defined normal androgen values in a group of age-, ethnicity-, and weight-comparable control women with regular menstrual cycles, no clinical signs of hyperandrogenemia, and normal glucose tolerance. A value >2 SD from the control mean is a widely accepted, albeit arbitrary, criterion in clinical chemistry and was used to define abnormal androgen levels. We have used this criterion to define hyperandrogenemia in all of our studies of PCOS since 1986 (10). These normal ranges have remained stable with ongoing control subject recruitment. There was some overlap in androgen levels between control women and affected relatives, but this overlap was less than the reported prevalence of polycystic ovaries as observed by ultrasound in normal women (9). Because PCOS is so common (10, 26), it is probable that some control women may have been hyperandrogenemic because they were members of PCOS families.
There is only one study of the actual prevalence of the endocrine syndrome of PCOS or hyperandrogenemia alone in an unselected population of women (26). These authors also found that they could phenotype women with a menstrual history, a physical examination, and a blood sample for androgen levels. Taken together, these studies indicate that women can be reliably phenotyped for hyperandrogenemia with a single blood sample if an appropriate normal control group is used (26). This will greatly expedite phenotyping reproductive-age women in PCOS kindreds for our planned linkage studies. Moreover, hyperandrogenemia with or without oligomenorrhea can be used as an additional phenotype in the linkage analysis, as it may reflect an underlying genetic defect in PCOS (1, 5). This will also substantially increase the number of affected sisters and, accordingly, the power of the linkage analysis (27).
The HA phenotype among PCOS sisters was similar biochemically to that found in ovulatory women with polycystic ovaries observed by ultrasound reported by a number of investigators (9, 28, 29). In our study, the HA sisters had a lower BMI than the PCOS sisters. This observation suggests that increased BMI is part of the PCOS phenotype. Whether increased adiposity contributes to the development of PCOS in genetically susceptible women will require further study. However, there is now considerable evidence to suggest that adiposity-related insulin resistance contributes to anovulation in women with PCOS (10, 14). The HA sisters also had an increased gravidity and parity compared with their PCOS sisters. This finding, combined with their history of regular menses, suggests they may be more fecund than their PCOS sisters.
In summary, this report of hyperandrogenemia with or without oligomenorrhea aggregating in PCOS families suggests that this finding has a genetic basis (5). The distribution of testosterone levels among sisters appeared to be bimodal, suggesting that this is a monogenic trait (20). Our findings provide insight into the heterogeneity of PCOS by indicating that several affected phenotypes can be present in one family, suggesting that the heterogeneity has a genetic basis (13), and could reflect variable expression of a monogenic trait or an oligogenic trait (27). We have shown that hyperandrogenemia can be reliably documented with a single blood sample. We propose that hyperandrogenemia be used as a phenotype in linkage studies to identify PCOS genes (1, 5, 27, 30).
Acknowledgments
We thank Dr. Richard Spielman for his critical review of the manuscript. This work was supported by The National Center for Infertility Research at University of Pennsylvania, Brigham and Women’s Hospital, and University of California at San Francisco (Grant U54 HD34449), the Core Endocrine Laboratory, Hershey Medical Center, Hershey, PA, and by Public Health Service Grants RO1 DK40605, K08 HDO118, and M01 RR10732, as well as grants from the American Diabetes Association and the Center for Research on Women and Newborn Health Foundation.
ABBREVIATIONS
- PCOS
polycystic ovary syndrome
- HA
hyperandrogenemia alone
- UA
unaffected
- T
testosterone
- uT
nonsex hormone-binding globulin-bound testosterone
- DHEAS
dehydroepiandrosterone sulfate
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- BMI
body mass index
Footnotes
Legro, R. S., Fox, J. & Dunaif, A., Tenth International Congress of Endocrinology, June 12–15, 1996, San Francisco, abstr. OR26-6.
Azziz, R. & Kahsar-Miller, M., Tenth International Congress on Hormonal Steroids, June, 1998, Quebec City, CA, abstr. 37.
References
- 1.Legro R S, Spielman R, Urbanek M, Driscoll D, Strauss J S, Dunaif A. Recent Prog Horm Res. 1998;53:217–256. [PubMed] [Google Scholar]
- 2.Franks S, Gharani N, Waterworth D, Batty S, White D, Williamson R, McCarthy M. Hum Reprod. 1997;12:2641–2648. doi: 10.1093/humrep/12.12.2641. [DOI] [PubMed] [Google Scholar]
- 3.Ferriman D, Purdie A W. Clin Endocrinol (Oxford) 1979;11:291–300. doi: 10.1111/j.1365-2265.1979.tb03077.x. [DOI] [PubMed] [Google Scholar]
- 4.Lunde O, Magnus P, Sandvik L, Hoglo S. Gynecol Obstet Invest. 1989;28:23–30. doi: 10.1159/000293493. [DOI] [PubMed] [Google Scholar]
- 5.Lander E S, Schork N J. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- 6.Jahanfar S, Eden J A, Warren P, Seppala M, Nguyen T V. Fertil Steril. 1995;63:478–486. [PubMed] [Google Scholar]
- 7.Carey A H, Chan K L, Short F, White D, Williamson R, Franks S. Clin Endocrinol. 1993;38:653–658. doi: 10.1111/j.1365-2265.1993.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 8.Adams J, Polson D W, Franks S. Br Med J. 1986;293:355–359. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polson D W, Adams J, Wadsworth J, Franks S. Lancet. 1988;1:870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- 10.Dunaif A. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 11.Hatch R, Rosenfield R L, Kim M H, Tredway D. Am J Obstet Gynecol. 1981;140:815–830. doi: 10.1016/0002-9378(81)90746-8. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO Expert Committee on Diabetes Mellitus: Second Report. World Health Organization, Geneva: Technical Report Series 626; 1980. [PubMed] [Google Scholar]
- 13.Zawadski J K, Dunaif A. In: The Polycystic Ovary Syndrome. Givens J R, Haseltine F, Merriam G R, editors. Oxford: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 14.Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. J Clin Endocrinol Metab. 1996;81:3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay R R, Dube J Y. Contraception. 1974;10:599–605. doi: 10.1016/0010-7824(74)90099-7. [DOI] [PubMed] [Google Scholar]
- 16.Givens J R, Andersen R N, Wiser W L, Fish S A. J Clin Endocrinol Metab. 1974;38:727–735. doi: 10.1210/jcem-38-5-727. [DOI] [PubMed] [Google Scholar]
- 17.Nestler J E, Jakubowicz D J. N Eng J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 18.Beer N A, Jacubowicz D J, Beer R M, Nestler J E. J Clin Endocrinol Metab. 1994;79:1077–1081. doi: 10.1210/jcem.79.4.7962276. [DOI] [PubMed] [Google Scholar]
- 19.Erhmann D A, Barnes R B, Rosenfield R L. Endocr Rev. 1995;16:322–353. doi: 10.1210/edrv-16-3-322. [DOI] [PubMed] [Google Scholar]
- 20.Weinshilboum R, Raymond F A. Am J Hum Genet. 1977;29:125–135. [PMC free article] [PubMed] [Google Scholar]
- 21.Santen R J, Bardin C W. J Clin Invest. 1973;52:2617–2628. doi: 10.1172/JCI107454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee P A, Migeon C J, Bias W B, Jones G S. Obstet Gynecol. 1987;69:259–264. [PubMed] [Google Scholar]
- 23.Hague W M, Adams J, Reeders S T, Peto T E, Jacobs H S. Clin Endocrinol. 1988;29:593–605. doi: 10.1111/j.1365-2265.1988.tb03707.x. [DOI] [PubMed] [Google Scholar]
- 24.Farquhar C M, Birdsall M, Manning P, Mitchell J M, France J T. Aust N Z J Obstet Gynecol. 1994;34:67–72. doi: 10.1111/j.1479-828x.1994.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 25.Norman R J, Masters S, Hague W. Fertil Steril. 1996;66:942–947. doi: 10.1016/s0015-0282(16)58687-7. [DOI] [PubMed] [Google Scholar]
- 26.Knochenhauer E S, Key T J, Kahsar-Miller M, Waggoner W, Boots L R, Azziz R. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, Shork N J. Diabetes. 1996;45:1–14. doi: 10.2337/diab.45.1.1. [DOI] [PubMed] [Google Scholar]
- 28.White D, Leigh A, Wilson C, Donaldson A, Franks S. Clin Endocrinol. 1995;42:475–481. doi: 10.1111/j.1365-2265.1995.tb02665.x. [DOI] [PubMed] [Google Scholar]
- 29.Adams J, Franks S, Polson D W, Mason H D, Abdulwahid U, Tucker M, Price J, Jacobs H S. Lancet. 1985;2:1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 30.Cox N J, Xiang K S, Fajans S S, Bell G I. Diabetes. 1992;41:401–407. doi: 10.2337/diab.41.4.401. [DOI] [PubMed] [Google Scholar]