Abstract
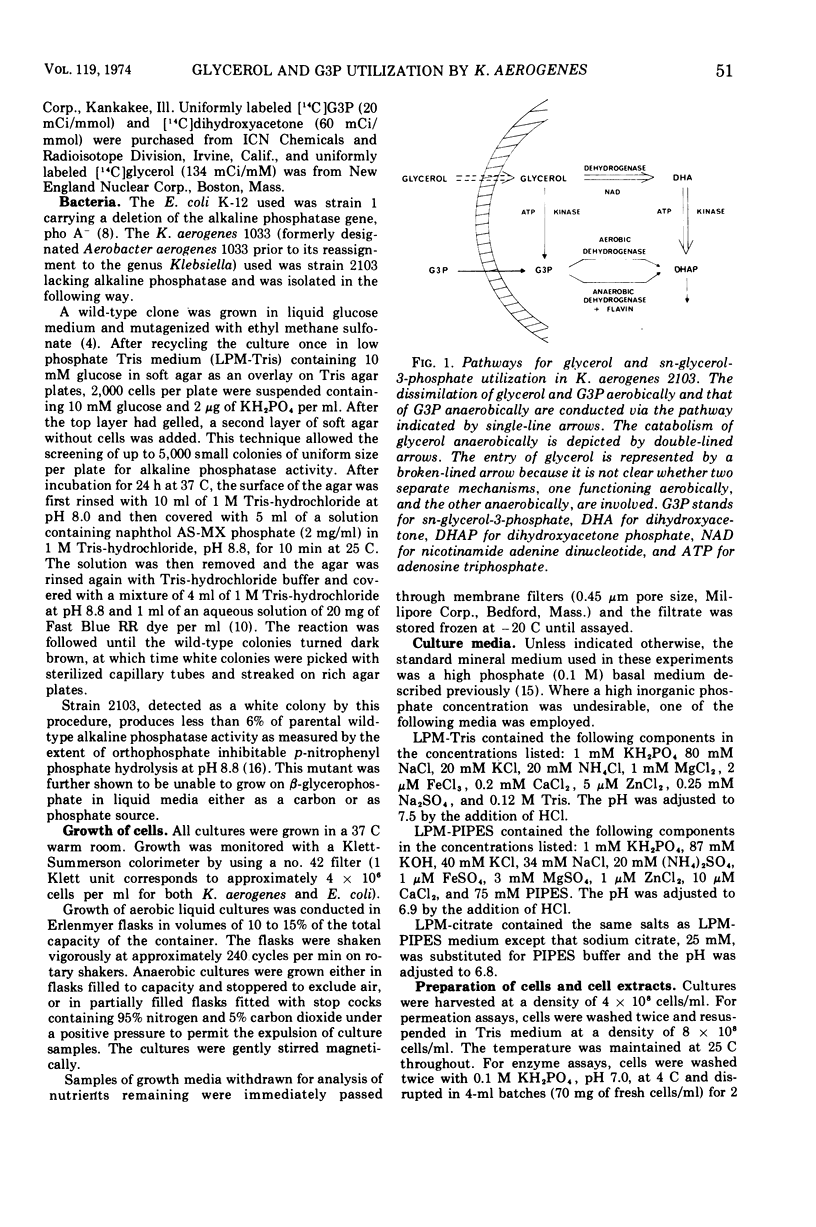
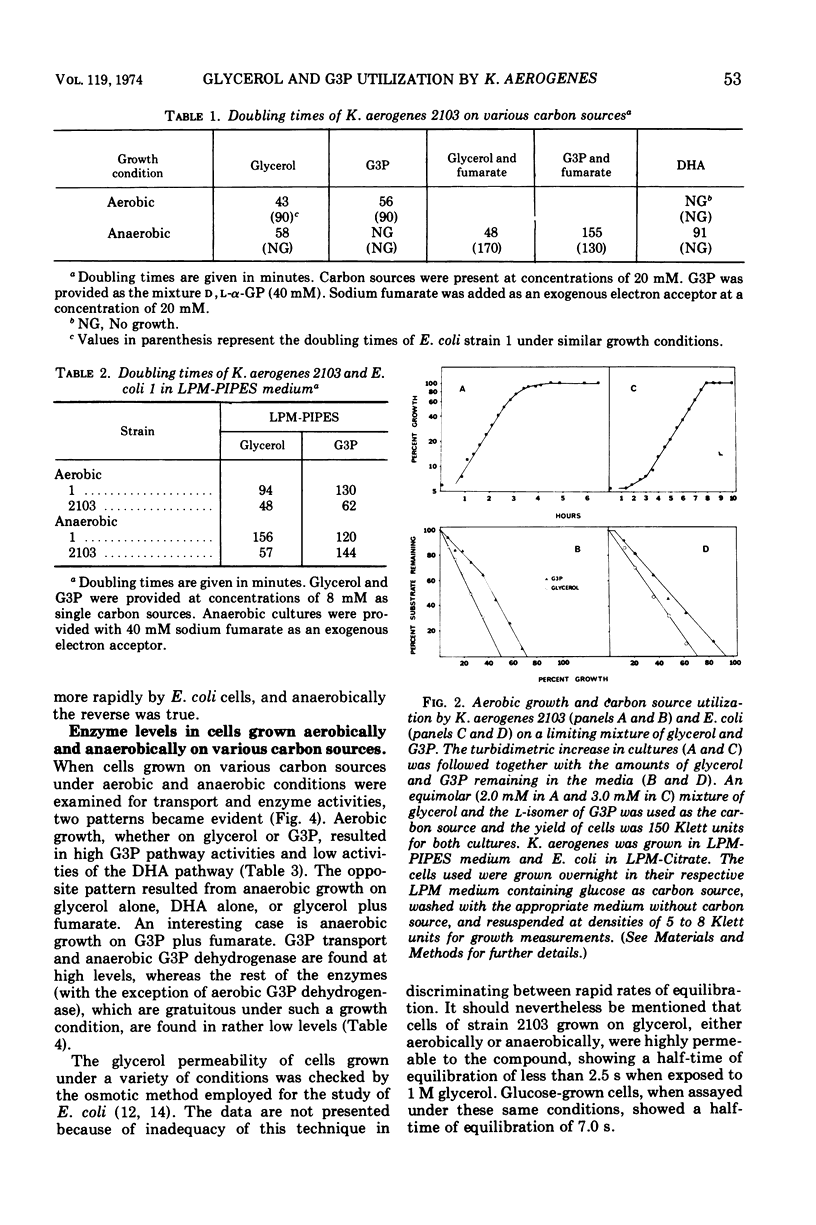
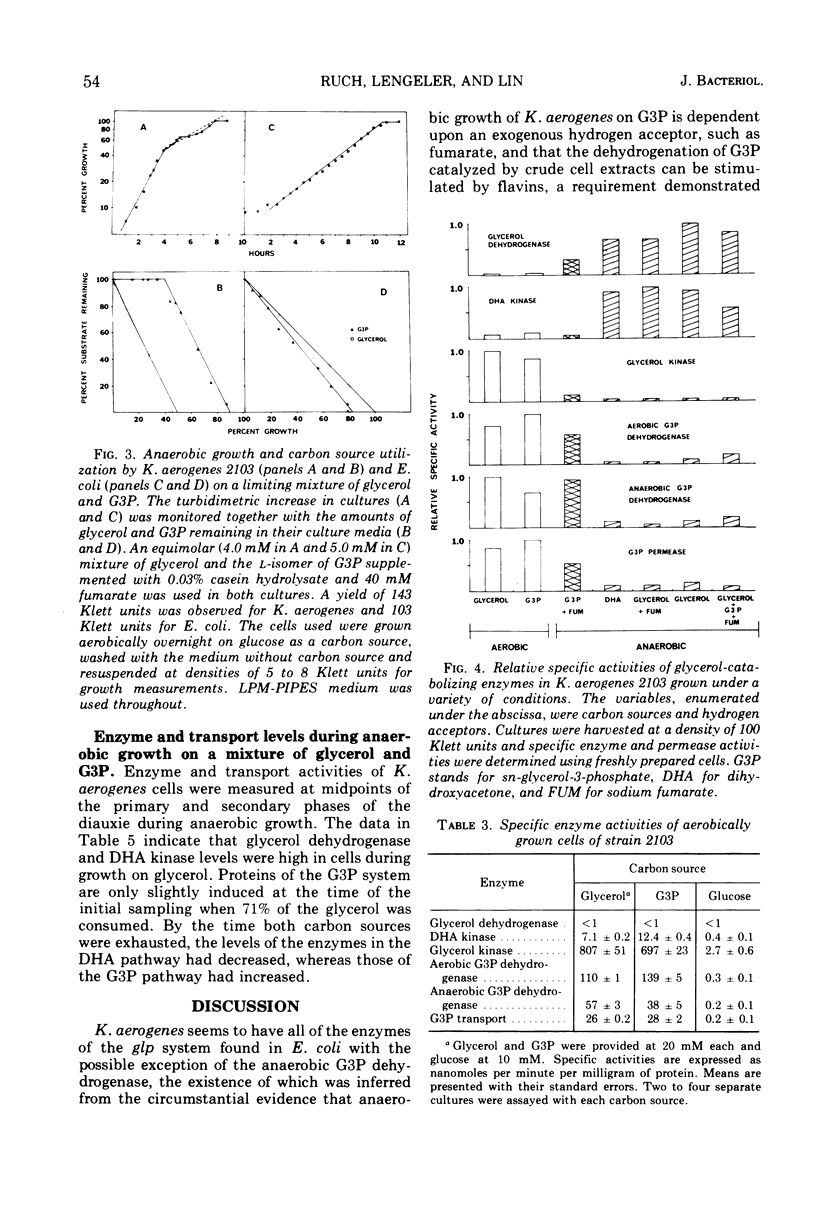
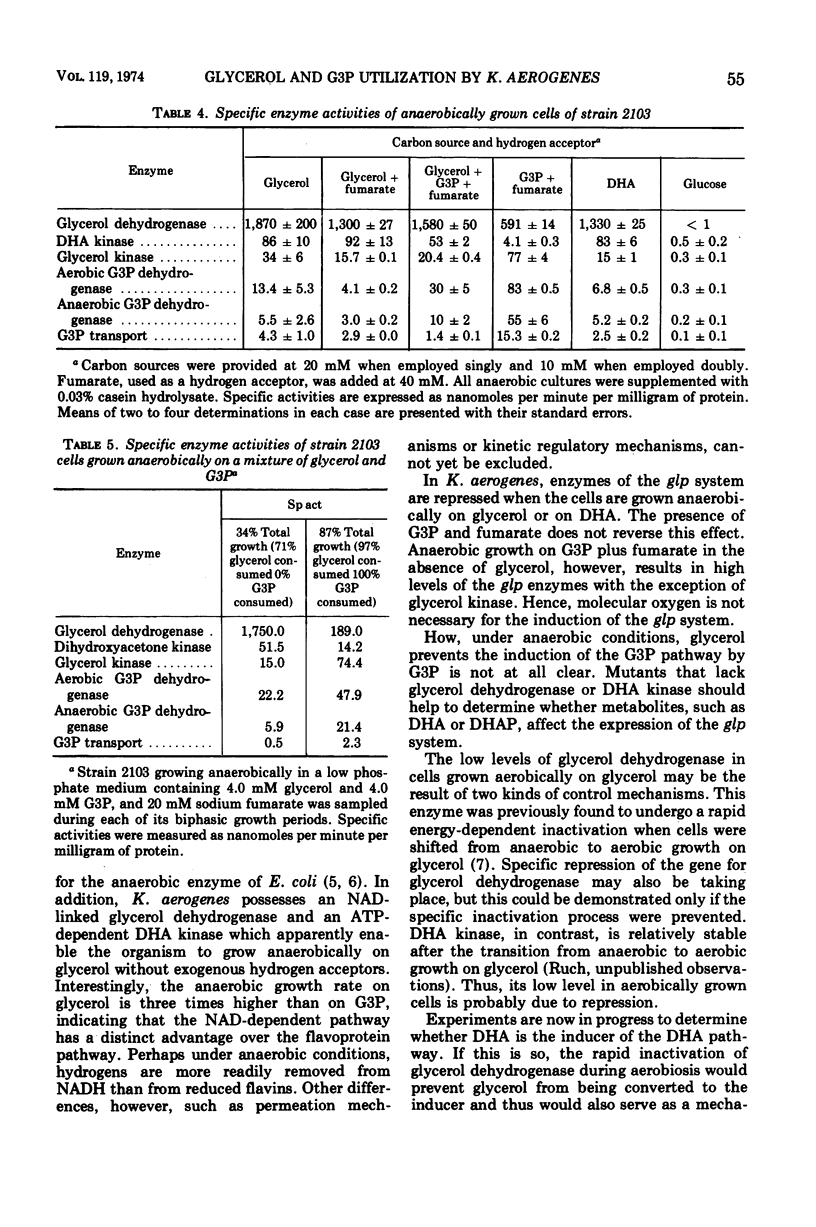
The utilization of glycerol as a carbon source for growth by Klebsiella aerogenes, strain 2103, involves separate aerobic (sn-glycerol-3-phosphate or G3P) and anaerobic (dihydroxyacetone or DHA) pathways of catabolism. Enzyme and transport activities of the aerobic pathway are elevated in cells grown under oxygenated conditions on glycerol or G3P. Anaerobic growth on G3P as carbon source requires the presence of an exogenous hydrogen acceptor such as fumarate; cells thus grown also are highly induced in the G3P pathway. Anaerobic growth on glycerol requires no exogenous hydrogen acceptors; cells thus grown are highly induced in the DHA pathway but almost uninduced in the G3P pathway and the addition of fumarate electron acceptors has no effect on the relative levels of the two pathways. When both glycerol and G3P are provided anaerobically with fumarate, the DHA pathway is still preferentially induced, which probably accounts for the exclusive utilization of glycerol until its exhaustion. These observations suggest the presence of a regulatory control of G3P pathway imposed by the operation of the DHA pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Freedberg W. B., Lin E. C. Three kinds of controls affecting the expression of the glp regulon in Escherichia coli. J Bacteriol. 1973 Sep;115(3):816–823. doi: 10.1128/jb.115.3.816-823.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Purification and properties of the flavine-stimulated anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli. J Bacteriol. 1972 Oct;112(1):539–547. doi: 10.1128/jb.112.1.539-547.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN E. C., LEVIN A. P., MAGASANIK B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960 Jun;235:1824–1829. [PubMed] [Google Scholar]
- MAGASANIK B., BROOKE M. S., KARIBIAN D. Metabolic pathways of glycerol dissimilation; a comparative study of two strains of Aerobacter aerogenes. J Bacteriol. 1953 Nov;66(5):611–619. doi: 10.1128/jb.66.5.611-619.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Messer W., Vielmetter W. High resolution colony staining for the detection of bacterial growth requirement mutants using naphthol azo-dye techniques. Biochem Biophys Res Commun. 1965 Oct 26;21(2):182–186. doi: 10.1016/0006-291x(65)90106-3. [DOI] [PubMed] [Google Scholar]
- RUSH D., KARIBIAN D., KARNOVSKY M. L., MAGASANIK B. Pathways of glycerol dissimilation in two strains of Aerobacter aerogenes; enzymatic and tracer studies. J Biol Chem. 1957 Jun;226(2):891–899. [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanno Y., Wilson T. H., Lin E. C. Control of permeation to glycerol in cells of Escherichia coli. Biochem Biophys Res Commun. 1968 Jul 26;32(2):344–349. doi: 10.1016/0006-291x(68)90392-6. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



