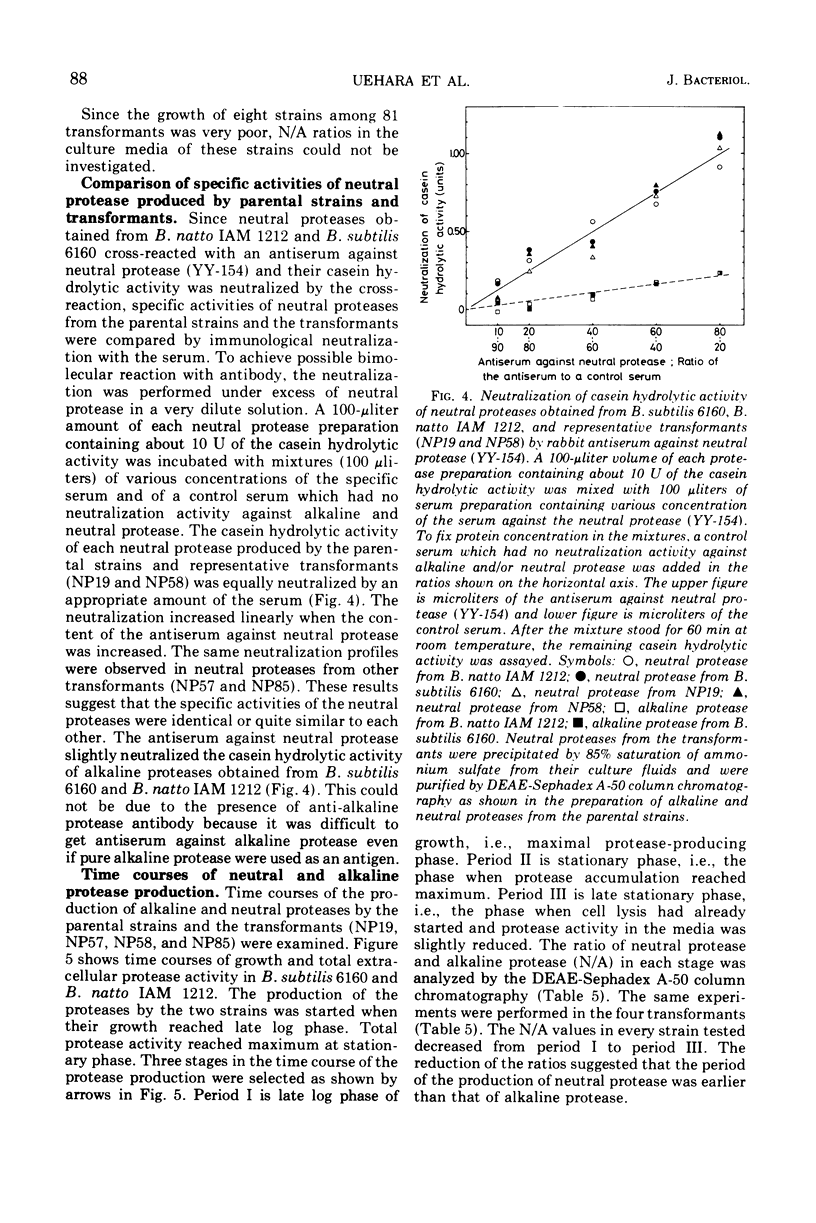
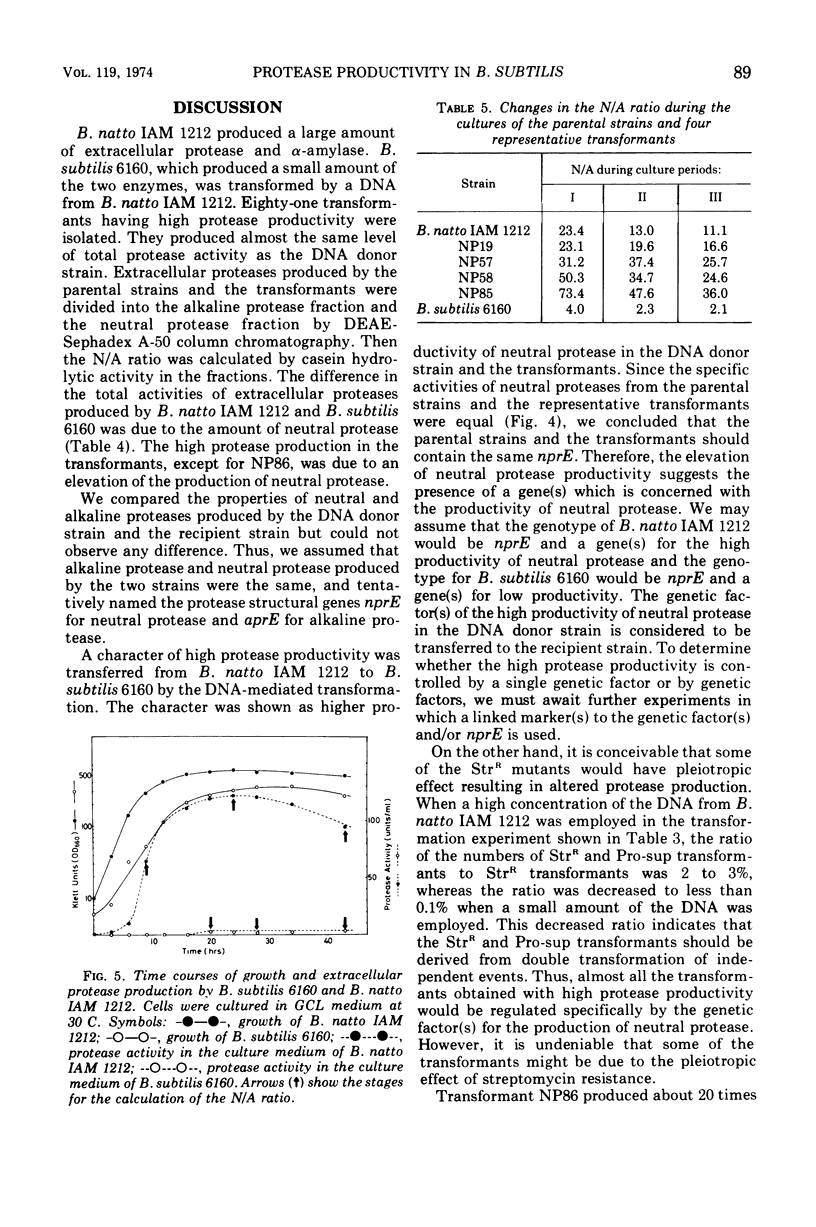
Abstract
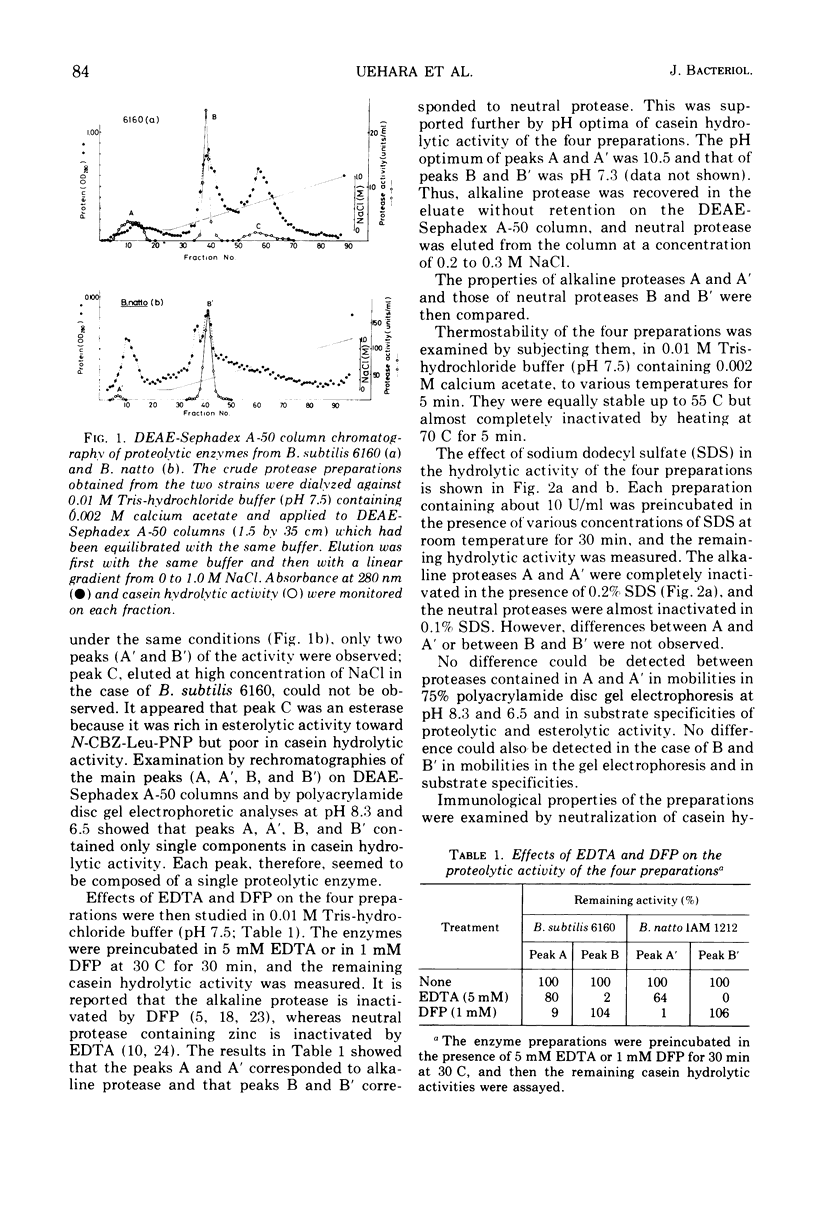
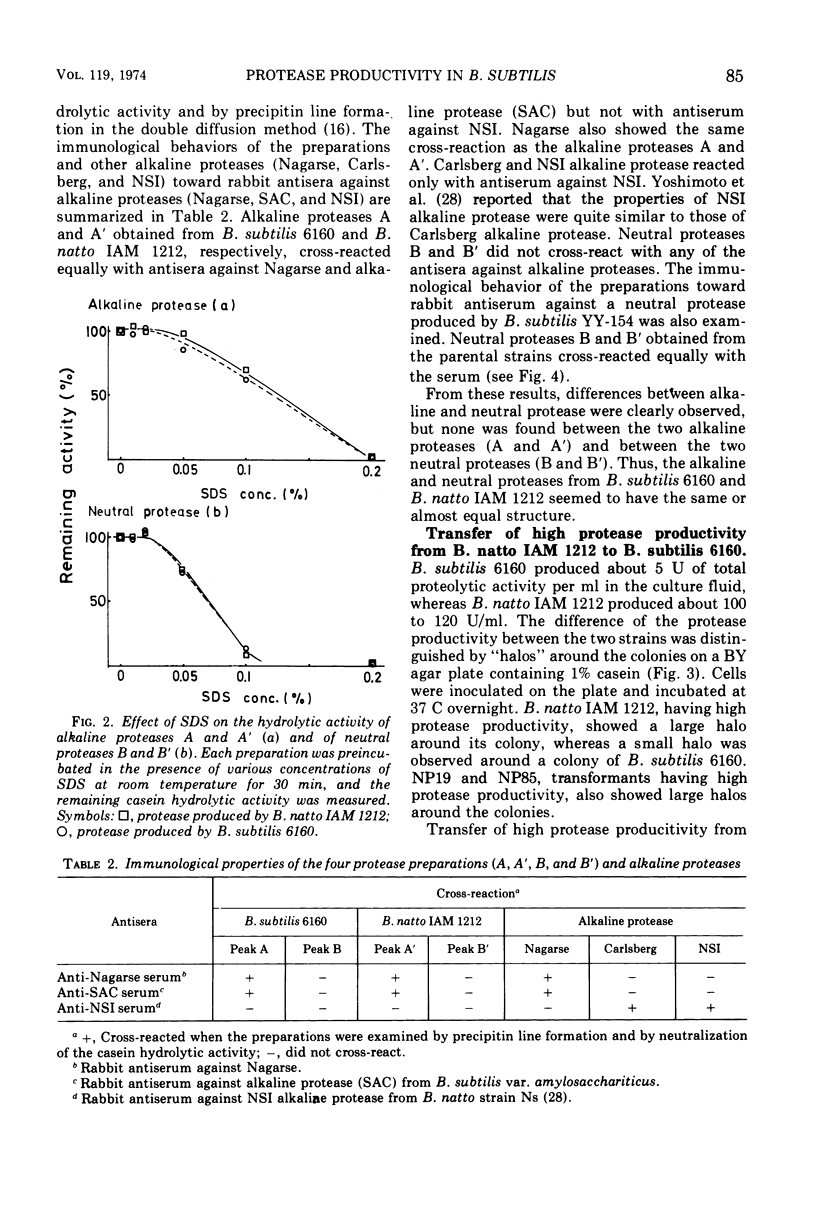
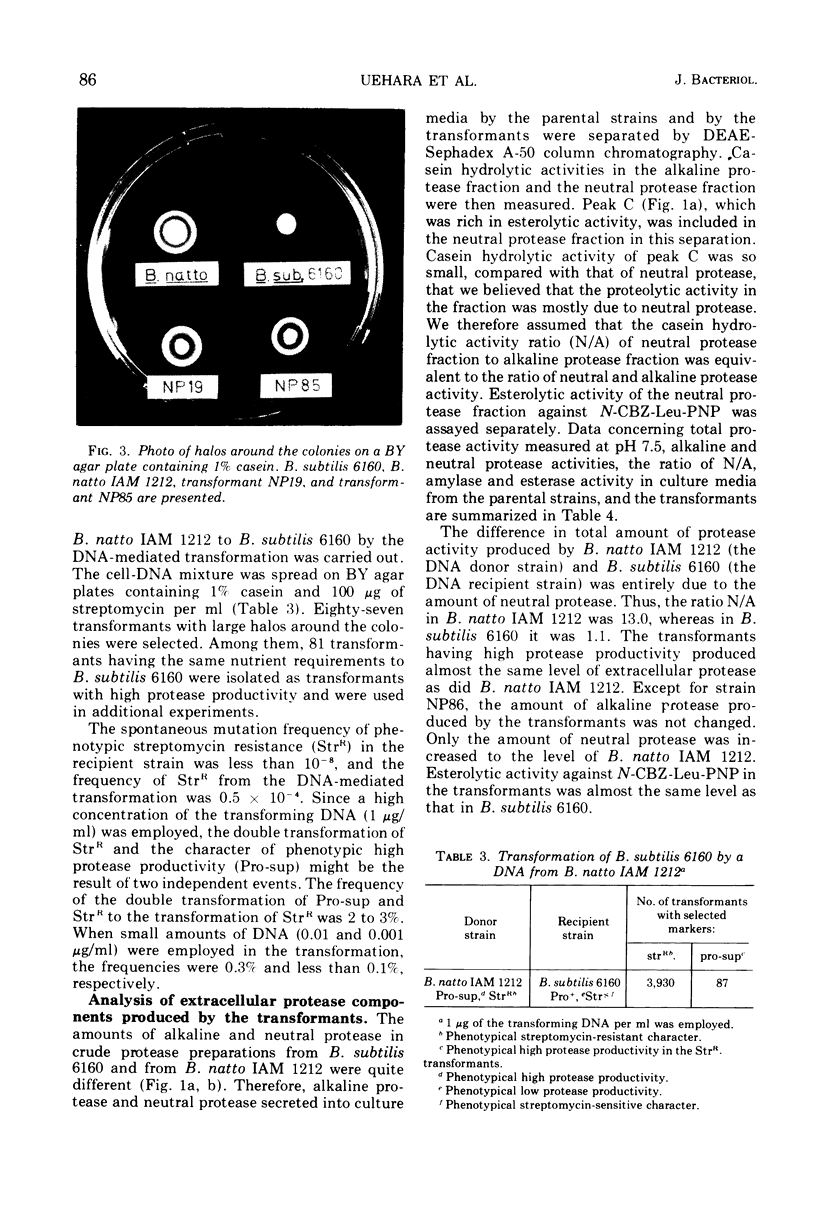
A transformable strain of Bacillus subtilis 6160, a derivative of B. subtilis 168, produces three kinds of casein hydrolytic enzymes (alkaline protease, neutral protease, and esterase) in a culture medium. B. natto IAM 1212 produces 15 to 20 times as much total proteolytic activity as does B. subtilis. Extracellular proteases produced by the two strains were separated into each enzyme fraction by diethylaminoethyl-Sephadex A-50 column chromatography. The difference in the total protease activities of extracellular proteases between the two strains was due to the amount of neutral protease. The ratios of neutral protease activity to alkaline protease activity (N/A) were 1.1 in B. subtilis 6160 and 13.0 in B. natto IAM 1212. Enzymological and immunological properties of alkaline protease and neutral protease obtained from the two strains were quite similar or identical, respectively. Specific activities measured by an immunological analysis of the two neutral proteases against casein were also equal. A genetic character of high protease productivity in B. natto IAM 1212 was transferred to B. subtilis 6160 by the deoxyribonucleic acid-mediated transformation. Among 73 transformants that acquired high protease productivity, 69 produced a higher amount of neutral protease and the ratios of N/A were changed to 15 to 60. Three other strains were transformed in the productivity of neutral protease and α-amylase simultaneously, and one showed considerable change in the production of alkaline protease and neutral protease. The specific activities (casein hydrolytic activities/enzyme molecules) of neutral proteases from the representative four transformants were equal to those of the two parental strains. These results suggested the presence of a specific gene(s) that participated in the productivity of neutral protease in B. subtilis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. An enzymatic and immunological comparison of two proteases from a transformable Bacillus subtilis with the "subtilisins". Arch Biochem Biophys. 1970 Jul;139(1):67–79. doi: 10.1016/0003-9861(70)90045-7. [DOI] [PubMed] [Google Scholar]
- Kadowaki K., Hosoda J., Maruo B. Effects of actinomycin D and 5-fluorouracil on the formation of enzymes in Bacillus subtilis. Biochim Biophys Acta. 1965 Jun 8;103(2):311–318. doi: 10.1016/0005-2787(65)90170-x. [DOI] [PubMed] [Google Scholar]
- MCCONN J. D., TSURU D., YASUNOBU K. T. BACILLUS SUBTILIS NEUTRAL PROTEINASE. I. A ZINC ENZYME OF HIGH SPECIFIC ACTIVITY. J Biol Chem. 1964 Nov;239:3706–3715. [PubMed] [Google Scholar]
- Markland F. S., Smith E. L. Subtilisin BPN. VII. Isolation of cyanogen bromide peptides and the complete amino acid sequence. J Biol Chem. 1967 Nov 25;242(22):5198–5211. [PubMed] [Google Scholar]
- Millet J. Characterization of proteinases excreted by Bacillus subtilis Marburg strain during sporulation. J Appl Bacteriol. 1970 Mar;33(1):207–219. doi: 10.1111/j.1365-2672.1970.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Comparison of alpha-chymotrypsin and subtilisin BPN': size and specificity of the active site. Biochem Biophys Res Commun. 1969 Apr 29;35(2):210–214. doi: 10.1016/0006-291x(69)90269-1. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Subtilisin BPN': kinetic study with oligopeptides. Arch Biochem Biophys. 1970 Jun;138(2):515–525. doi: 10.1016/0003-9861(70)90376-0. [DOI] [PubMed] [Google Scholar]
- OISHI M., TAKAHASHI H., MARUO B. Intracellular alpha-amylase in Bacillus subtilis. J Bacteriol. 1963 Jan;85:246–247. doi: 10.1128/jb.85.1.246-247.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPAPORT H. P., RIGGSBY W. S., HOLDEN D. A. A BACILLUS SUBTILIS PROTEINASE. I. PRODUCTION, PURIFICATION, AND CHARACTERIZATION OF A PROTEINASE FROM A TRANSFORMABLE STRAIN OF BACILLUS SUBTILIS. J Biol Chem. 1965 Jan;240:78–86. [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Smith E. L., DeLange R. J., Evans W. H., Landon M., Markland F. S. Subtilisin Carlsberg. V. The complete sequence; comparison with subtilisin BPN'; evolutionary relationships. J Biol Chem. 1968 May 10;243(9):2184–2191. [PubMed] [Google Scholar]
- Smith E. L., Markland F. S., Kasper C. B., DeLange R. J., Landon M., Evans W. H. The complete amino acid sequence of two types of subtilisin, BPN' and Carlsberg. J Biol Chem. 1966 Dec 25;241(24):5974–5976. [PubMed] [Google Scholar]
- Wright C. S., Alden R. A., Kraut J. Structure of subtilisin BPN' at 2.5 angström resolution. Nature. 1969 Jan 18;221(5177):235–242. doi: 10.1038/221235a0. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Maruo B. Membrane mutation related to the production of extracellular -amylase and protease in bacillus subtilis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):765–770. doi: 10.1016/0006-291x(73)91310-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. Temperature-sensitive mutants of Bacillus subtilis. I. Multiforked replication and sequential transfer of DNA by a temperature-sensitive mutant. Proc Natl Acad Sci U S A. 1970 Jan;65(1):206–213. doi: 10.1073/pnas.65.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T., Fukumoto J., Tsuru D. Studies on bacterial proteases. Some enzymatic and physiochemical properties of the alkaline protease from Bacillus natto. Int J Protein Res. 1971;3(5):285–295. [PubMed] [Google Scholar]
- Yuki S. On the gene controlling the rate of amylase production in Bacillus subtilis. Biochem Biophys Res Commun. 1968 Apr 19;31(2):182–187. doi: 10.1016/0006-291x(68)90727-4. [DOI] [PubMed] [Google Scholar]




