Abstract
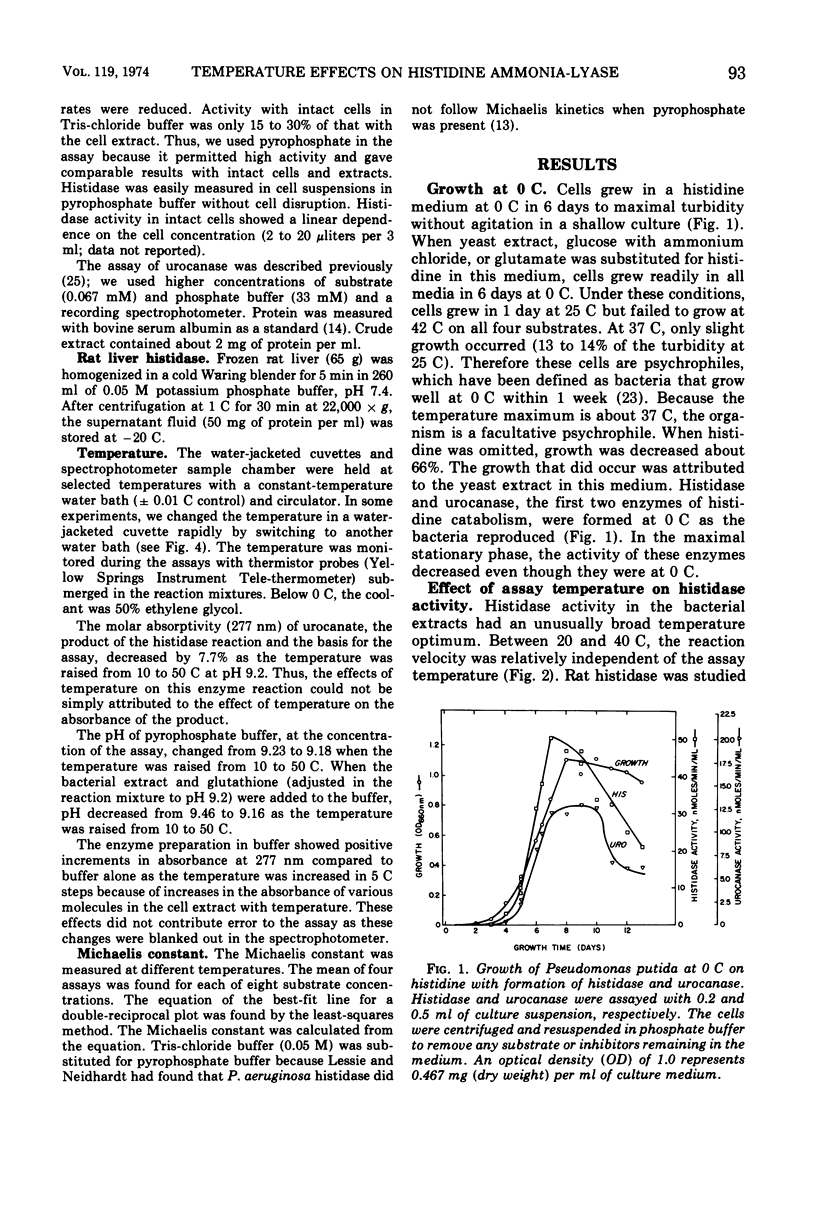
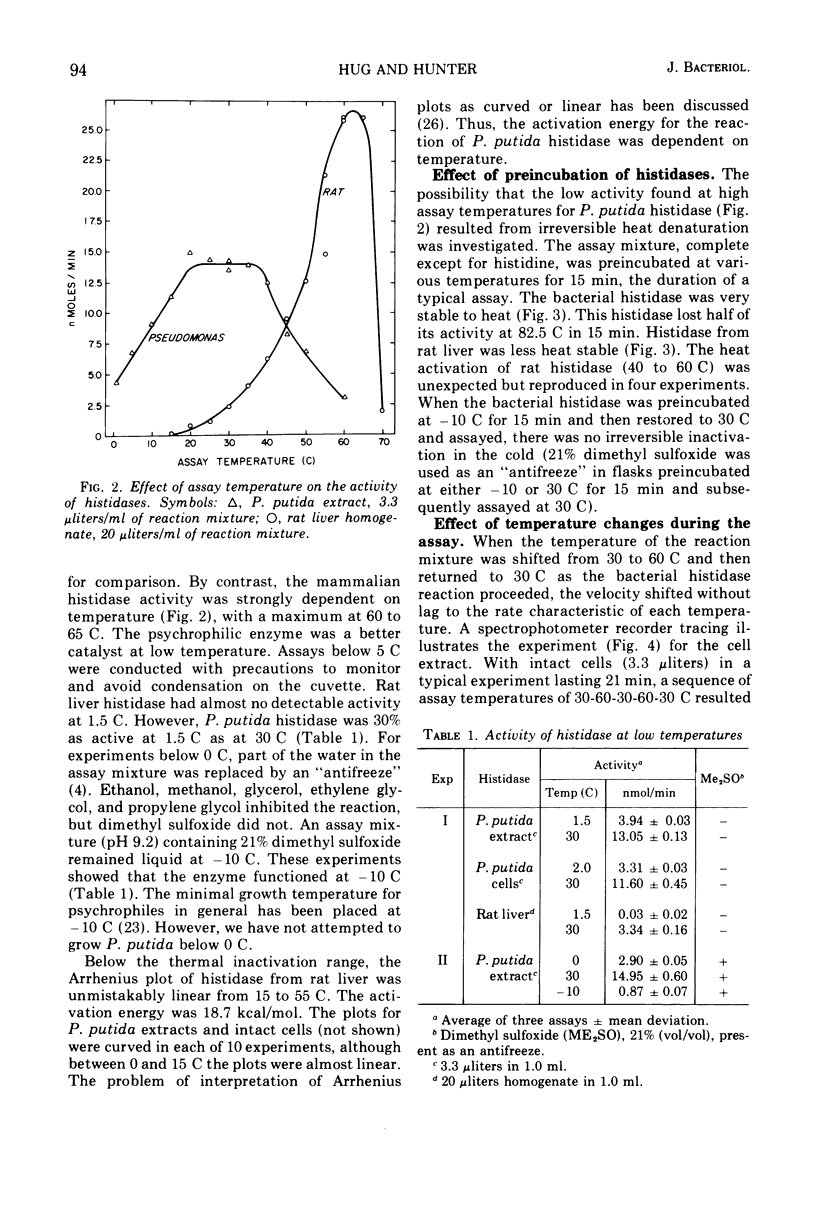
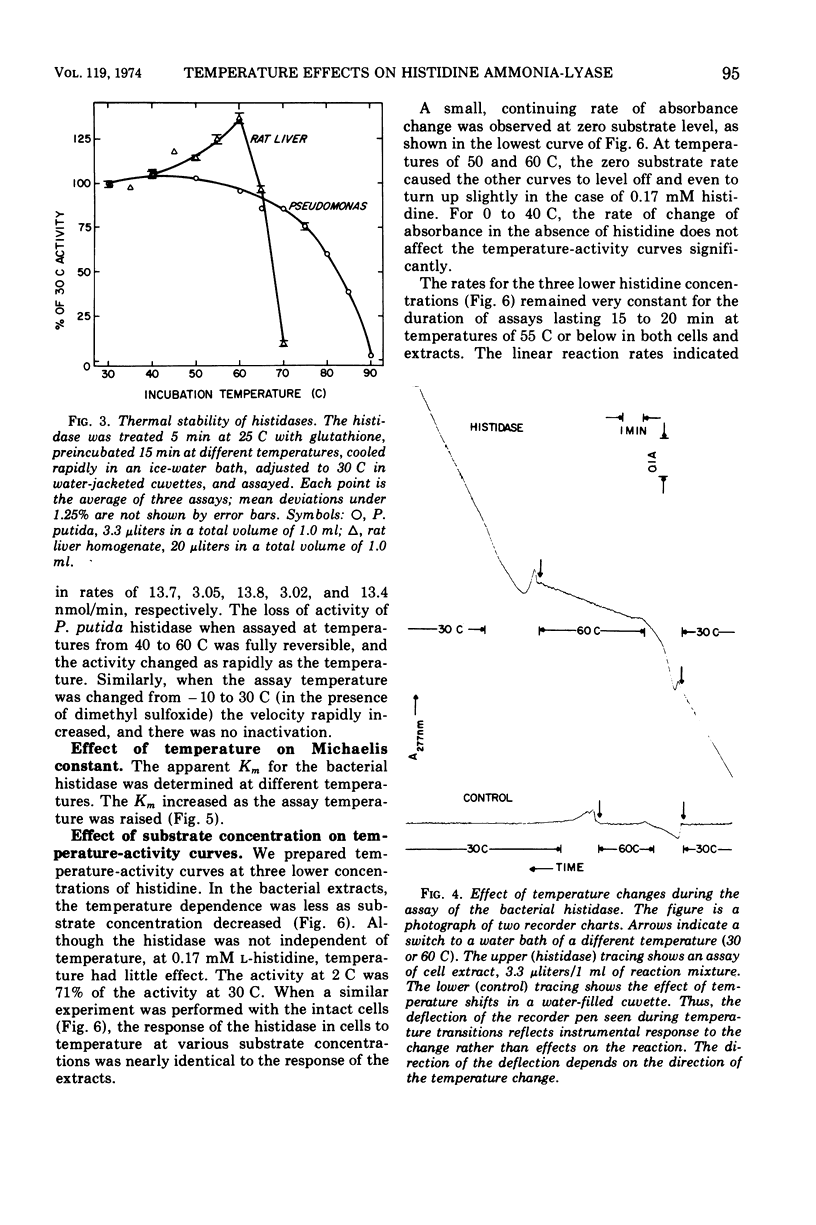
Pseudomonas putida was able to grow at 0 C in a complex medium containing l-histidine and to synthesize histidine ammonia-lyase and urocanase. The activity of the former enzyme was assessed between −10 and 60 C in cells and in cell extracts. Activity was maximal from 20 to 35 C. Below 20 C, activity decreased with temperature but, significantly, the enzyme exhibited 30% of its maximal activity at 1.5 C. The temperature response was similar in both intact cells and cell extracts, which indicated that the cell membrane did not significantly limit the entry of histidine at low temperature. Above and below the maximal temperature range, the reduced activity was not caused by irreversible inactivation, as shown by preincubation experiments. Also, when the temperature was rapidly changed from 60 to 30 C during an assay, the reaction rate increased abruptly to the full 30 C activity without a lag. This demonstrated the rapid reversibility of inactivation. The apparent Michaelis constant increased with temperature. As the substrate concentration was decreased, the enzyme activity became less dependent on temperature. The efficiency of substrate entry and catalysis near 0 C are factors in the ability of this facultative psychrophile to grow in a histidine medium at 0 C.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J., Hochachka P. W. Functional significance of isoenzymes in thermal acclimatization. Acetylcholinesterase from trout brain. Biochem J. 1970 Mar;116(5):883–887. doi: 10.1042/bj1160883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrisch H. W. Temperature and the regulation of enzyme activity in poikilotherms. Fructose diphosphatase from migrating salmon. Biochem J. 1969 Dec;115(4):687–696. doi: 10.1042/bj1150687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debey P., Balny C., Douzou P. Enzyme assay in microsomes below zero degrees. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2633–2636. doi: 10.1073/pnas.70.9.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne D. J., Barns E. M., Parker L., Fuchs F. The effect of temperature on actomyosin. Biochim Biophys Acta. 1972 Apr 20;267(1):190–202. doi: 10.1016/0005-2728(72)90150-8. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Lewis J. K. Enzyme variants in thermal acclimation. Trout liver citrate synthases. J Biol Chem. 1970 Dec 25;245(24):6567–6573. [PubMed] [Google Scholar]
- Hochachka P. W., Lewis J. K. Interacting effects of pH and temperature on the K m values for fish tissue lactate dehydrogenases. Comp Biochem Physiol B. 1971 Aug 15;39(4):925–933. doi: 10.1016/0305-0491(71)90116-7. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Somero G. N. The adaptation of enzymes to temperature. Comp Biochem Physiol. 1968 Dec;27(3):659–668. doi: 10.1016/0010-406x(68)90605-1. [DOI] [PubMed] [Google Scholar]
- Hug D. H., Hunter J. K. Effect of temperature on urocanase from a psychrophile, Pseudomonas putida. Biochemistry. 1974 Mar 26;13(7):1427–1431. doi: 10.1021/bi00704a017. [DOI] [PubMed] [Google Scholar]
- Hug D. H., Roth D., Hunter J. Regulation of histidine catabolism by succinate in Pseudomonas putida. J Bacteriol. 1968 Aug;96(2):396–402. doi: 10.1128/jb.96.2.396-402.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T. G., Neidhardt F. C. Formation and operation of the histidine-degrading pathway in Pseudomonas aeruginosa. J Bacteriol. 1967 Jun;93(6):1800–1810. doi: 10.1128/jb.93.6.1800-1810.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon T. W., Hochlachka P. W. Temperature and enzyme activity in poikilotherms. Isocitrate dehydrogenases in rainbow-trout liver. Biochem J. 1971 Aug;123(5):695–705. doi: 10.1042/bj1230695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule M. R. The effect of temperature on the kinetics of adenosine diphosphoglucose pyrophosphorylase from Rhodospirillum rubrum. Biochemistry. 1971 Nov 23;10(24):4509–4517. doi: 10.1021/bi00800a026. [DOI] [PubMed] [Google Scholar]
- Purohit K., Stokes J. L. Heat-labile enzymes in a psychrophilic bacterium. J Bacteriol. 1967 Jan;93(1):199–206. doi: 10.1128/jb.93.1.199-206.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist R. G., Stokes J. L. Comparative effect of temperature on the induced synthesis of hydrogenase and enzymes of the benzoate oxidation system in psychrophilic and mesophilic bacteria. Can J Microbiol. 1972 Aug;18(8):1233–1239. doi: 10.1139/m72-191. [DOI] [PubMed] [Google Scholar]
- Rechler M. M. The purification and characterization of L-histidine ammonia-lyse (Pseudomonas). J Biol Chem. 1969 Feb 25;244(4):551–559. [PubMed] [Google Scholar]
- Singleton R., Jr, Amelunxen R. E. Proteins from thermophilic microorganisms. Bacteriol Rev. 1973 Sep;37(3):320–342. doi: 10.1128/br.37.3.320-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero G. N., Hochachka P. W. Isoenzymes and short-term temperature compensation in poikilotherms: activation of lactate dehydrogenase isoenzymes by temperature decreases. Nature. 1969 Jul 12;223(5202):194–195. doi: 10.1038/223194a0. [DOI] [PubMed] [Google Scholar]
- Svenneby G. Time and temperature dependent activation of pig brain glutaminase. J Neurochem. 1972 Jan;19(1):165–174. doi: 10.1111/j.1471-4159.1972.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Talsky G. The anomalous temperature dependence of enzyme-catatlyzed reactions. Angew Chem Int Ed Engl. 1971 Aug;10(8):548–554. doi: 10.1002/anie.197105481. [DOI] [PubMed] [Google Scholar]
- Vidal M. C., Cazzulo J. J. CO 2 -fixing enzymes in a marine psychophile. J Bacteriol. 1972 Oct;112(1):427–433. doi: 10.1128/jb.112.1.427-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLENFELS K., MALHOTRA O. P. Galactosidases. Adv Carbohydr Chem. 1961;16:239–298. doi: 10.1016/s0096-5332(08)60264-7. [DOI] [PubMed] [Google Scholar]



