Abstract
Human β-defensins (HBDs) are antimicrobial peptides that may play a role in mucosal defense. Diminished activity of these peptides has been implicated in the pathogenesis of cystic fibrosis (CF) lung disease. We show that HBD-1 and HBD-2 mRNAs are expressed in excised surface and submucosal gland epithelia from non-CF and CF patients. The pro-inflammatory cytokine interleukin-1β stimulated the expression of HBD-2 but not HBD-1 mRNA and peptide in primary cultures of airway epithelia. HBD-1 was found in bronchoalveolar lavage (BAL) fluid from normal volunteers, CF patients, and patients with inflammatory lung diseases, whereas HBD-2 was detected in BAL fluid from patients with CF or inflammatory lung diseases, but not in normal volunteers. Both HBD-1 and HBD-2 were found in BAL fluid in concentrations of several ng/ml, and both recombinant peptides showed salt-sensitive bactericidal activity. These data suggest that in the lung HBD-2 expression is induced by inflammation, whereas HBD-1 may serve as a defense in the absence of inflammation.
Defensins are broad spectrum antimicrobial peptide products of neutrophils (α-defensins) and epithelia (β-defensins) (1, 2). Two β-defensins have been isolated in humans, β-defensin-1 (HBD-1), originally isolated from blood filtrate (3) and expressed in the urogenital tract (4), and human β-defensin-2 (HBD-2), a peptide identified in skin that is induced in keratinocytes in response to infection and inflammation (5). Although there is evidence that the mRNAs for both HBD-1 and HBD-2 are expressed in the lung (5–7), neither peptide has been detected in airway surface liquid (ASL) of in vitro model systems or in human bronchoalveolar lavage (BAL) fluid. Furthermore, it is not known whether β-defensin production is deficient in diseases such as cystic fibrosis (CF) that are characterized by chronic infection. Recent studies have identified β-defensin expression at mucosal surfaces in human tissues, where they may play a role in innate defenses (2, 4, 6, 7).
Pulmonary mucosal defenses are impaired in CF, a disease characterized by chronic airway infection in the absence of any demonstrable systemic immune defect (2, 8, 9). Several studies have suggested that diminished activity of antimicrobial peptides such as the β-defensins may contribute to the inability of CF epithelia to clear bacteria from their surface (2, 9). Although there is evidence that the mRNAs for both HBD-1 and -2 are expressed in the lung (5–7), neither peptide has been detected in ASL of in vitro model systems or in human BAL fluid. In this study we utilized epithelia and BAL fluid from non-CF and CF patients to address three main questions: (i) Are HBD-1 and -2 messages expressed similarly by CF and non-CF epithelia in the basal state and after stimulation with proinflammatory stimuli? (ii) Can the β-defensin peptides be detected in ASL in vitro and in vivo? (iii) Do the HBD-1 and -2 peptides exhibit antimicrobial activity and is this activity inhibited by the increased ASL NaCl concentrations postulated to occur in CF?
MATERIALS AND METHODS
Cell Cultures.
Human CF epithelia were obtained from nasal polyp surgical specimens, and non-CF epithelial cells were dissociated from human trachea and bronchus autopsy specimens. CF epithelia were genotyped for CFTR (CF transmembrane conductance regulator) mutations (Genzyme) and shown to be ΔF508/ΔF508. Epithelia from each specimen were studied in Ussing chambers and were found to lack apical membrane Cl− permeability. The epithelia were seeded on collagen-coated semipermeable membranes (surface area 0.6 cm2) and grown at the air–liquid interface as previously described (10–12). The basolateral surface of the membrane was in contact with DMEM and Ham’s F-12 media (1:1), 2% Ultroser G (Sepracor, Marlborough, MA) and antibiotics (penicillin at 100 units/ml, streptomycin at 100 μg/ml, gentamicin at 40 μg/ml, and fluconazole at 2 mg/ml). The differentiated epithelia were examined with scanning electron microscopy and shown to be ciliated and to secrete an amorphous material on the apical surface (10). The study was approved by the Institutional Review Board of the University of Iowa.
Detection of HBD-1 and HBD-2 mRNA.
RNA was isolated from cell cultures as previously described (8) by using single-step extraction with guanidine thiocyanate/phenol/chloroform. To assess the effect of an inflammatory stimulus on β-defensin mRNA and protein expression, some epithelia were incubated with 100 ng/ml recombinant human interleukin-1β (IL-1β; R & D Systems). β-Defensin mRNA was detected by the ribonuclease protection assay (RPA) method, using 20 μg of non-CF or CF airway epithelial RNA as previously described (7).
In Situ Hybridization.
We followed methods that were previously used to localize HBD-1 mRNA in urogenital tissues (4). Sense and antisense α-[35S]thio-UTP, α-[35S]thio-CTP (Amersham) double-labeled HBD-1 and HBD-2 riboprobes were prepared from linearized cDNA plasmids and hybridized with frozen sections of CF (n = 3) and non-CF (n = 4) lung tissue according to published methods (13). CF samples were obtained at the time of lung transplantation and non-CF samples were obtained from patients who died of nonrespiratory causes. The sections were coated with NTB-2 autoradiography emulsion (Eastman Kodak) diluted 1:1 in distilled H2O, air dried, and exposed for 2–12 weeks at 4°C before being developed. Sections were counterstained with toluidine blue, and signal was localized by using bright-field and dark-field microscopy.
Preparation of Recombinant HBD-1 and HBD-2 Peptides and Polyclonal Rabbit Antisera.
Recombinant HBD-1 and HBD-2 peptides were made by using an insect cell/baculovirus expression system as described previously (4, 14). Briefly, the cDNAs for HBD-1 and HBD-2 were transfected into baculovirus by means of the BacPAK9 transfer vector. The baculovirus was then used to infect Sf21 insect cells, and recombinant HBD-1 and HBD-2 were purified from the culture media (4, 14). HBD-1 and HBD-2 polyclonal antisera were prepared by coupling the recombinant peptides to ovalbumin and immunizing rabbits intradermally (4). Despite the homology of the peptide antigens, we found no cross-reactivity between the two antibodies when tested against the recombinant peptides by Western blotting.
Immunodetection of HBD-1 and HBD-2 Peptides.
β-Defensins were detected by acid/urea/polyacrylamide gel electrophoresis and Western blotting with rabbit antisera to HBD-1 or HBD-2 diluted 1:1000 in TBS (Tris-buffered saline) (4). A horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:20,000 dilution) was used as the secondary antibody.
The apical surfaces of cultured epithelial sheets were washed with sterile water (100 μl each time). The tissue source of the cultures was as follows: six CF (all nasal polyp) and five non-CF (one nasal turbinate, one trachea, three bronchus). The washings were lyophilized and resuspended in 17 mM NH4OAc, pH 4, with 0.1% Triton X-100 in 10% of the original volume. To recover any peptide that was tightly adherent or intracellular, cells were lysed in 17 mM NH4OAc, pH 4, with 0.1% Triton X-100 after the apical surface washings.
Human BAL fluid from normal adult volunteers (n = 6), CF patients (n = 6), and patients with inflammatory lung diseases (n = 18) were also screened for β-defensin peptides by Western immunoblotting. The normal volunteers had no clinical or laboratory evidence of lung disease. The CF patients all had clinical diagnoses of CF characterized by malabsorption, lung disease, and positive sweat tests. The inflammatory lung disease samples were obtained from patients with biopsy-proven cases of sarcoidosis, idiopathic pulmonary fibrosis, and other restrictive lung diseases, followed by the University of Iowa Specialized Center of Research in Interstitial Lung Diseases. Samples (2–10 ml) were sonicated and cationic polypeptides were extracted by mixing with 50 μl of 50% CM-Prep (Bio-Rad) slurry per ml of BAL as described previously for other biological fluids (4). The gel beads were batch eluted with acetic acid and the pooled eluates were lyophilized and resuspended in 50 μl of 1% acetic acid, and 1–10 μl was analyzed by Western immunoblotting as described for other biological fluids (4). Concentrations in ASL and BAL specimens were estimated by comparison of the immunoblot band intensity with the signal from recombinant peptide. In estimating ASL concentrations from BAL samples we assumed the ASL levels were at least 100-fold more concentrated than the BAL levels. As a control, lysozyme levels were measured for each BAL sample and were within the range of published concentrations, assuming a 100-fold dilution.
Antimicrobial Activity Assays.
Two assays were used to test the antimicrobial activity of recombinant HBD-1 and HBD-2: a quantitative luminescence assay and a conventional colony-forming unit assay. The luminescence assay employs Escherichia coli DH5α containing the luminescence plasmid pCGLS1 (15) and allows quantitative assessment of bactericidal activity. Luminescence is an energy-requiring activity that is directly related to bacterial viability under the conditions used (data not shown). Bacteria were grown at 30°C in Luria–Bertani medium, centrifuged, and resuspended (107 cells per ml) in 10 mM potassium phosphate, pH 7.2, with 1% Luria–Bertani medium. Bacteria (106) were then incubated with recombinant peptides in the buffer described above plus NaCl in concentrations between 0 and 200 mM in 96-well plates (Optiplate, Packard Instruments) for 4 hr, and luminescence was measured with a microtiter dish luminometer (LucyI, Anthos LabTech, Straussberg, Austria).
Colony-forming unit (CFU) assays of β-defensin antimicrobial activity against Pseudomonas aeruginosa PAO1 were performed as follows: Bacteria were grown in trypticase soy broth at 37°C. Cells from exponential-phase cultures were suspended to a density of 2 × 105 CFU/ml in sterile 10 mM potassium phosphate buffer, pH 7.2, and incubated with serial dilutions of recombinant β-defensin, for 4 h at 37°C. The bacteria were then spread on agar plates and the colonies were counted after 24 h at 37°C.
RESULTS
Expression of HBD-1 and HBD-2 mRNAs in Airway Epithelia.
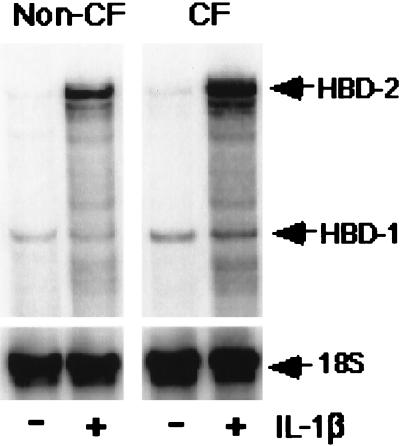
To assess how a relevant pro-inflammatory stimulus affects the expression of both β-defensins, epithelia were exposed to IL-1β for 24 h. Ribonuclease protection assays showed that unstimulated epithelia expressed HBD-1 and HBD-2 mRNAs at low levels. Stimulation with IL-1β increased the abundance of HBD-2, but not HBD-1 mRNA (Fig. 1). Time course experiments (data not shown) indicated that HBD-2 mRNA increased within 1.5 h of exposure to IL-1β and reached a peak at 36 h, when it was 10-fold higher than it was prior to IL-1β treatment. Results with CF were similar to those described for non-CF epithelia. Experiments were repeated in triplicate, and similar results were obtained.
Figure 1.
Expression of HBD-1 and HBD-2 mRNAs in cultured normal and CF airway epithelia measured by using the ribonuclease protection assay. Cells were studied under basal conditions (−) and after 24 h of IL-1β treatment (+; 100 ng/ml). For both non-CF and CF specimens, constitutive HBD-1 mRNA expression was detected and was unchanged by IL-1β treatment. In contrast, the mRNA for HBD-2 was markedly induced after IL-1β treatment. 18S rRNA was used as an internal control to standardize the mRNA signal. Shown is a representative assay from three separate experiments with similar results.
Localization of β-Defensin mRNA by in Situ Hybridization.
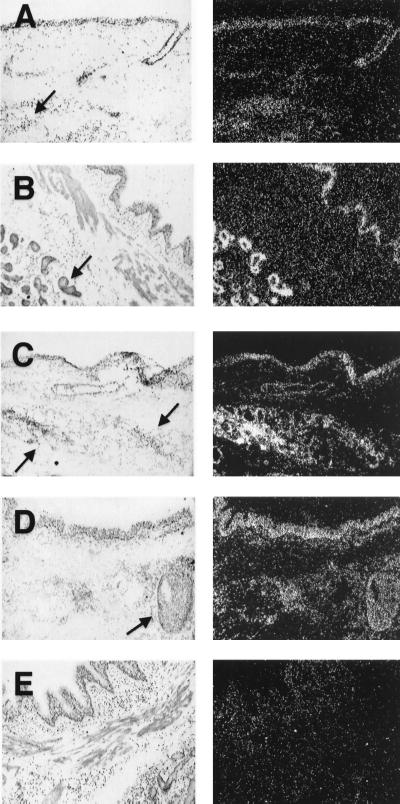
Analysis with an antisense HBD-1 riboprobe showed the mRNA to be expressed diffusely throughout the surface and submucosal gland epithelia of both CF and non-CF airway tissue samples (Fig. 2). A subpopulation of submucosal gland epithelia morphologically compatible with serous cells showed higher levels of HBD-1 mRNA. The expression in serous cells was best appreciated in the CF specimen. There was no obvious difference in the overall intensity of the HBD-1 mRNA signal between CF and non-CF airways; however, an exposure of the photographic emulsion for 8–11 weeks was required to detect the HBD-1 mRNA signal, suggesting that the overall abundance of the message was low in both CF and non-CF. Analysis with an antisense HBD-2 riboprobe showed the HBD-2 mRNA was also expressed by most cell types in the surface and submucosal gland epithelia of CF and non-CF airways (Fig. 2). The signal was apparent after a 6- to 8-week exposure to photographic emulsion. The signal intensity for both probes in the gas exchange regions, blood vessels, and mesenchymal tissues of the lung was not appreciably greater than background, and no specific signal was detected with the sense HBD-1 or HBD-2 riboprobes, confirming the specificity of the antisense signal. Thus, the HBD-1 and -2 mRNAs are expressed in the surface epithelia and submucosal glands, and the pattern of expression in CF is similar to that in normal lung.
Figure 2.
Localization of β-defensin mRNA expression in normal and CF airway by in situ hybridization. For each pair of images the left-hand panel is a bright-field image and the right-hand panel is dark-field. (A) HBD-1 is expressed in normal human bronchus in surface and submucosal gland epithelia (antisense probe, 8-week exposure). (B) HBD-1 is also expressed in CF bronchus in surface and submucosal gland epithelia (antisense probe, 11-week exposure). (C) HBD-2 is expressed in normal human bronchus in surface and submucosal gland epithelia (antisense probe, 8-week exposure). (D) HBD-2 is expressed in CF bronchus in surface and submucosal gland epithelia (antisense probe, 8-week exposure). (E) Representative control sense riboprobe for HBD-1 with nonspecific background. Similar results were found with the HBD-2 sense riboprobe. Arrows denote submucosal glands. (×10.)
Detection of β-Defensin Peptides in ASL.
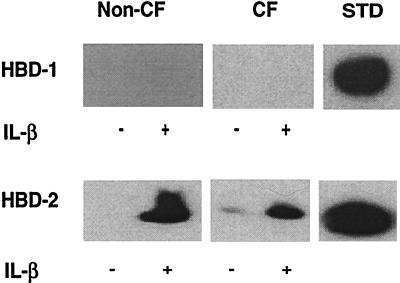
Airway epithelia grown at the air–liquid interface are well suited for the study of ASL components, as the cells differentiate and have distinct apical and basolateral compartments. The apical surface of the cells was washed, and pooled washings were examined by immunoblotting with HBD-1 and HBD-2 antisera. Despite concentration of the ASL constituents 10-fold by lyophilization, HBD-1 protein was not detected in washings from either unstimulated or IL-1β-stimulated airway epithelial sheets (Fig. 3 Upper). To investigate the possibility that HBD-1 was secreted, but tightly bound to the epithelia, we examined whole cell lysates for the presence of HBD-1 and again found no detectable peptide (data not shown). By comparison to the standard (5 ng of recombinant HBD-1 peptide), we estimate that this assay should detect HBD-1 in amounts as low as 1 ng/ml.
Figure 3.
Detection of HBD-1 and HBD-2 proteins in non-CF and CF ASL by acid/urea/PAGE and immunoblotting. Epithelia were cultured with (+) and without (−) stimulation. (Upper) Comparison of HBD-1 peptide abundance in non-CF and CF epithelia. No HBD-1 peptide was found in washings from non-CF or CF epithelia, but 5 ng of recombinant HBD-1 peptide (STD) was easily detected. No HBD-1 was detected in the cell lysates (not shown). (Lower) Comparison of HBD-2 abundance in non-CF and CF epithelia. The apical surface was washed with water to remove accumulated peptide prior to IL-1β treatment. HBD-2 peptide was easily detected in the washings from non-CF and CF epithelia. Little peptide was recovered with the subsequent NH4OAc wash, indicating good recovery of protein with aqueous washes (not shown). HBD-2 was also detected in the cell lysate (not shown). With IL-1β stimulation, protein recovery increased in all fractions. Results for CF cells are qualitatively similar to those from cultured non-CF airway epithelia. Control was 7.5 ng of HBD-2. Representative results are shown.
In contrast to the findings with HBD-1, we detected the HBD-2 peptide in surface washings from non-CF and CF epithelia (Fig. 3 Lower). Washings from unstimulated non-CF epithelia that had been cultured for several weeks without disturbing the apical surface contained a basal level of HBD-2 (not shown). The presence of HBD-2 in the ASL from unstimulated epithelia may represent its accumulation because of basal mRNA expression and suggests that the HBD-2 peptide is stable in ASL. Both non-CF and CF epithelia secreted HBD-2 in response to IL-1β (Fig. 3). The apical surface was washed with water to remove accumulated peptide prior to IL-1β treatment. After an incubation period of 24 h, HBD-2 peptide was readily detected in the washings from IL-1β-stimulated non-CF and CF epithelia, whereas little peptide was present in the washings from nonstimulated controls. In addition to the band that comigrated with the 41 amino acid HBD-2 standard, a faint species of reduced mobility was also detected (Fig. 3, + IL-1β samples). By comparison to a recombinant HBD-2 standard, we estimated the concentration of HBD-2 in ASL of cultured, IL-1β-stimulated non-CF epithelia to be in the range of 8–10 μg/ml or approximately 2.0 μM (Fig. 3). This assumes an ASL depth of 20 μm and surface area of 0.6 cm2 for each epithelial culture. The amount of HBD-2 peptide produced by CF epithelia (obtained from excised nasal polyps) was more variable. This observation may reflect the disease state of the donor patients or the region of the airway from which the specimens were obtained. From our limited study, we cannot determine whether there are quantitative differences in the amounts of β-defensin in normal and CF ASL. Further study of larger numbers of CF and non-CF epithelia of approximately the same age and from the same airway region is needed.
Detection of β-Defensin Peptides in BAL Fluid.
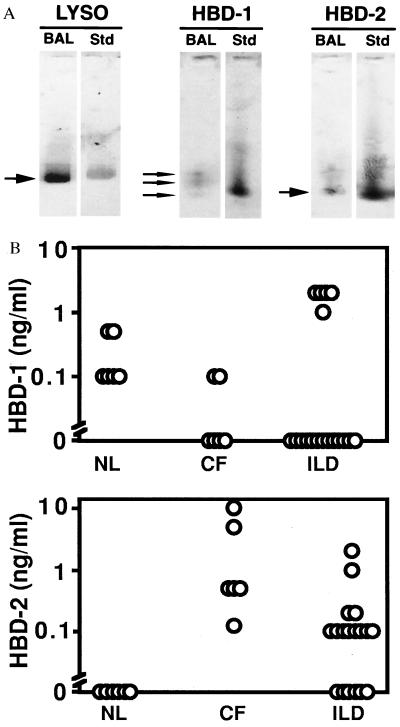
For β-defensins to play a role in the mucosal defenses of the lung they must be present in biologically relevant concentrations. We studied human BAL fluid from normal subjects (n = 6), patients with CF (n = 6), and patients with chronic lung diseases associated with inflammation (“ILD,” n = 18, includes sarcoid, idiopathic pulmonary fibrosis, and restrictive lung disease) by immunoblotting with antisera to HBD-1 and HBD-2. As a control, lysozyme antiserum was used. Representative immunoblot results are shown in Fig. 4A. Lysozyme was present in BAL from all patient groups in concentrations from 0.75 to 10 μg/ml (data not shown). Assuming a 100-fold dilution of ASL in the BAL sample, these concentrations are consistent with published data (16). HBD-1 was present in BAL fluid from some individuals in all three groups in concentrations up to 2 ng/ml based on the peptide standards (Fig. 4B). Three species of HBD-1 were detected (Fig. 4A). The finding of more than one band immunoreactive with HBD-1 antisera is similar to recent studies of HBD-1 peptide expression in the urogenital tract, where four species ranging in length from 36 to 47 amino acids were detected in urine (4). Unlike HBD-1, the HBD-2 peptide was not detected in any BAL sample from 6 normal subjects (Fig. 4B). However, HBD-2 was detected in BAL fluid from all 6 CF patients (range 0.1 to 10 ng/ml) and in 12/18 inflammatory lung disease patients (range 10 to 100 ng/ml). When HBD-2 was present, a single species was identified.
Figure 4.
Concentration of β-defensin peptides in BAL fluid measured by Western immunoblotting. Human BAL samples were obtained and analyzed by acid/urea/PAGE and Western immunoblotting with specific antisera for HBD-1 and HBD-2. Samples from non-CF (NL), CF, and patients with inflammatory lung diseases (ILD) were studied. (A) Representative immunoblot results for lysozyme (LYSO), HBD-1, and HBD-2 (BAL, patient sample; Std, protein standard). (B) BAL β-defensin concentrations determined by comparison to peptide standards. Each open circle represents the value from a single patient sample. HBD-1 was detectable in some samples from all three groups but there were no statistically significant differences among the groups. HBD-2 was detected only in BAL from patients with CF or ILD (P < 0.05 for both groups compared with non-CF by ANOVA).
Antimicrobial Activity of Recombinant HBD-1 and HBD-2.
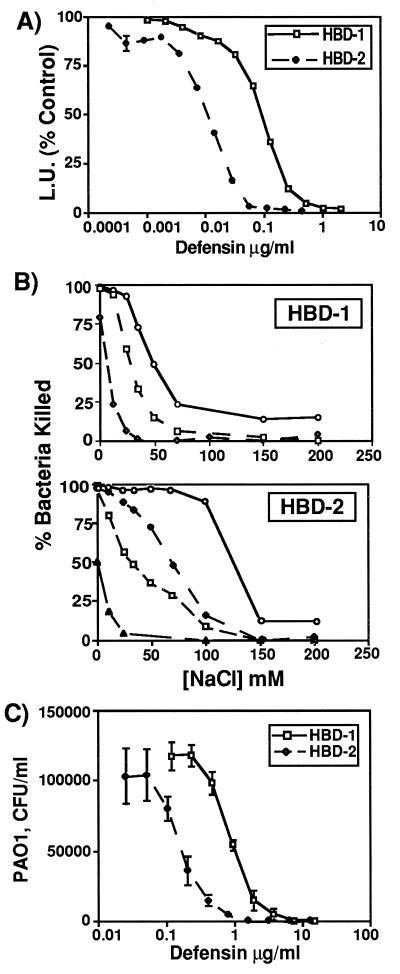
To assess the antimicrobial activity of β-defensin peptides, we produced and purified recombinant peptides (see Materials and Methods) and tested their ability to kill E. coli DH5α and P. aeruginosa PAO1. While both HBD-1 and HBD-2 killed E. coli in a dose-dependent manner, HBD-2 was approximately 10-fold more potent than HBD-1 (Fig. 5A). To determine how the antimicrobial activity of the β-defensins was affected by NaCl, we tested several doses of these peptides at NaCl concentrations ranging from 0 to 200 mM (Fig. 5B). The antimicrobial activity of both HBD-1 and HBD-2 was inhibited by salt, in a concentration-dependent manner. Interestingly, we found that the salt sensitivity of the β-defensin peptides could be overcome by increasing their concentrations. For HBD-1, increasing the concentration 10-fold (from 1.2 μg/ml to 12 μg/ml) shifted the point at which half the killing activity was lost from about 20 mM NaCl to about 50 mM NaCl. For HBD-2, a 10-fold increase in concentration (from 0.05 to 0.5 μg/ml) changed the point at which half the killing activity was lost from about 40 mM NaCl to about 150 mM NaCl. Both defensins also had antimicrobial activity against P. aeruginosa PAO1, and again, HBD-2 was approximately 10-fold more potent than HBD-1 (Fig. 5C). The dose required to kill 50% of the P. aeruginosa was 100 ng/ml and 1 μg/ml for HBD-2 and HBD-1, respectively.
Figure 5.
Antimicrobial activity and salt sensitivity of recombinant HBD-1 and HBD-2 peptides. (A) E. coli luminescence assay. HBD-1 and HBD-2 both kill E. coli in a dose-dependent fashion. The activity of HBD-2 was ≈10-fold greater than that of HBD-1. Each point represents mean ± SE for triplicate samples. (B) Salt sensitivity of HBD-1 and HBD-2. Increasing concentrations of NaCl inhibit the activity of both peptides against E. coli. The degree of inhibition by salt is also dependent on the concentration of peptide. For HBD-1, ○ = 12 μg/ml, □ = 6 μg/ml, and ⋄ = 1.2 μg/ml. For HBD-2, ○ = 0.5 μg/ml, ⋄ = 0.1 μg/ml, □ = 0.05 μg/ml, and ▵, 0.01 μg/ml. Results were replicated twice. (C) Colony-forming unit (CFU) assay for killing of P. aeruginosa by HBD-1 and HBD-2. Each point represents mean ± SE for triplicate samples.
DISCUSSION
We have presented evidence that the mRNAs for both HBD-1 and HBD-2 are expressed at low levels in human airway epithelia (Fig. 1). In situ hybridization showed that both mRNAs are diffusely expressed throughout the surface and submucosal gland epithelia of both CF and non-CF airways in a similar pattern (Fig. 2). IL-1β increased HBD-2 but not HBD-1 mRNA levels in cultured airway epithelia (Fig. 1). This pattern of expression was reflected in BAL studies, where HBD-1 peptide was detected in normals and patients with inflammatory lung diseases, whereas HBD-2 was present only in samples from patients with diseases associated with inflammation (Fig. 4). The presence of the peptides in BAL specimens supports the view that they contribute to the mucosal defenses of the lung. In addition, the antimicrobial activity of HBD-1 and HBD-2 was inhibited by salt in vitro (Fig. 5), suggesting that elevations in the ASL NaCl concentration could impair their function in vivo.
Taken together, these and other studies suggest that both of the β-defensins play important, although somewhat different, roles in the mucosal defense of the lung. HBD-1 appears to be expressed in the presence or absence of inflammation. Zhao et al. (6) found that HBD-1 mRNA expression was not induced by a wide variety of mediators, including combinations of cytokines, lipopolysaccharide, and phorbol ester. Goldman et al. (2) reported high-level expression of HBD-1 message in a tracheal xenograft model that had not been exposed to any mediators of inflammation. Although the HBD-1 peptide was not detected in the ASL in that study, they found almost complete ablation of the antimicrobial activity in ASL by antisense inhibition of HBD-1 (2). We also found expression of the HBD-1 message in cultured human airway epithelia, but we were unable to detect the peptide in washings or cell lysates from these cultures, perhaps because its concentration is below the limit of detection of our antibody. However, we did find the HBD-1 peptide in some BAL samples from normal individuals, CF patients, and patients with inflammatory lung diseases. While IL-1β did not influence HBD-1 mRNA or protein expression in our studies, it is possible that other regulatory stimuli exist. For example, HBD-1 peptide concentration is increased in the urine during pregnancy (4). The variability of HBD-1 in BAL fluids from patients not only with CF but also with other inflammatory lung diseases suggests that CFTR is not directly involved in HBD-1 production or secretion.
HBD-2 may play a somewhat different role in the innate defense of the lung. HBD-2 appears to be the human homolog of TAP and LAP, β-defensins expressed by bovine airway epithelia that are induced by lipopolysaccharide, tumor necrosis factor α, bovine pathogens, and mechanical injury (17–20). Like TAP and unlike HBD-1, the 5′ flanking region of HBD-2 contains consensus binding sequences for NF-κB, suggesting that these peptides are transcriptionally regulated by immunological stimuli such as IL-1β (21, 22). IL-1β is an important signaling molecule in the lung, especially during infection. It is rapidly secreted by resident macrophages in a site-specific manner after exposure to bacteria in both CF and non-CF lungs (23). It is known to stimulate cell-mediated immune responses, such as the activation of B and T lymphocytes, and the induction of second messengers and cytokines. Our finding that IL-1β induced HBD-2 secretion by airway epithelia suggests that in the lung, a first-response cytokine stimulates both cell-mediated and mucosal defense mechanisms in a coordinated manner. Consistent with this idea, we found the HBD-2 peptide only in the BAL fluid from patients with conditions associated with infection or inflammation, suggesting that HBD-2 may be more important once constitutive defenses have been breached and inflammatory signals are generated. Further studies with a larger number of patient samples are needed to better define the range of ASL β-defensin concentrations in health and disease states.
Both of the recombinant β-defensins exhibited potent antimicrobial activity against Gram-negative bacteria, including the CF-associated pathogen P. aeruginosa (Fig. 5). The activity of HBD-2 exceeds that of other α- and β-defensins, including HBD-1, by approximately 10-fold. The α-defensins and HBD-1 generally exhibit activity in the 1–50 μg/ml range (1, 2, 24). In our experiments HBD-2 killed 50% of P. aeruginosa PAO1 at about 100 ng/ml. A similar killing effect with HBD-1 required about 1 μg/ml. We estimate the concentrations of HBD-1 and HBD-2 in BAL fluid to be as high as 1 μg/ml (assuming a 100-fold dilution of the ASL as a result of the lavage). Thus, for HBD-2 there was sufficient peptide in some samples to fall within the range of antimicrobial activity. The concentrations of HBD-1 peptide we measured in BAL fall below those generally considered bactericidal. Thus, it is possible that HBD-1 may not play an important role in mucosal defenses of the lung. However, the peptides may be concentrated at the sites of their secretion onto the negatively charged epithelial surface and the actual β-defensin concentrations at the site of airway infection might be significantly higher because of local regulatory stimuli. Synergistic effects of epithelial defensins with more abundant antimicrobial polypeptides such as lysozyme may also be important for the activity of ASL against certain pathogens.
The antimicrobial activities of both HBD-1 and HBD-2 were inhibited by NaCl. The defensins are thought to exert their antimicrobial activity by interacting with membranes of metabolically active bacteria, perhaps by forming pores, and causing membrane disruption (24). Why their antimicrobial activity, and that of most of the other cationic peptides, is inhibited by salt is not known with certainty. The most likely possibility is that simple charge competition inhibits the initial interactions between a cationic peptide and the negatively charged bacterial membrane (25). Interestingly, we observed that the inhibitory effect of NaCl on β-defensin peptide activity depended on both the concentration of NaCl and the concentration of the peptide. This finding suggests that even at high salt concentrations, HBD-1 and HBD-2 may exhibit antimicrobial activity if their concentration is sufficiently high.
Our findings may be relevant to the pathogenesis of CF lung disease. CF patients are unable to effectively eliminate pathogenic bacteria from the surface of their airways (8). Interestingly, while CF airways are almost continuously infected, patients seldom develop invasive tissue infections in the lungs or elsewhere, and thus their immune defects appear confined to the airway surface (8). In our studies, we found no differences in β-defensin mRNA expression by CF and non-CF epithelia. It is difficult to make strict quantitative comparisons of HBD-2 peptide production by CF and non-CF epithelia from our in vitro studies without analysis of a greater number of samples from the same regions of the airway. However, the finding of HBD-2 peptide in BAL fluids from patients with CF, but not in normals, makes it unlikely that there is a primary defect in β-defensin production or secretion in CF. The antimicrobial function of both peptides was inhibited by salt. Although questions remain (26, 27), there is mounting evidence that normal ASL has a low NaCl concentration and CF a somewhat higher NaCl concentration (2, 28–30). If these native antibiotics function in a salt-sensitive manner in vivo, the antimicrobial potency of ASL may be inhibited in CF. The persistence of the bacteria in ASL may trigger the influx and activation of neutrophils, whose secretory products eventually contribute to lung damage.
Acknowledgments
We thank Linda McCarter for her assistance with immunoblot analyses. We thank Terry Grunst, Laura O’Brien, Christina Park, and Guoshun Wang for technical assistance, David Schwartz and Gary Hunninghake for BAL samples, Pary Weber, Phil Karp, and the CF Cell Culture Core for airway epithelia. This work was supported in part by the National Institutes of Health (HL42385 MJW), the Cystic Fibrosis Foundation (P.B.M 97ZO, T.G. 97Z0, E.P.G.97ZO), the Children’s Miracle Network Telethon, and the Howard Hughes Medical Institute. P.B.M. is the recipient of a Career Investigator Award from the American Lung Association.
ABBREVIATIONS
- ASL
airway surface liquid
- CF
cystic fibrosis
- HBD-1
human β-defensin-1
- HBD-2
human β-defensin-2
- IL-1β
interleukin 1β
- BAL
bronchoalveolar lavage
References
- 1.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman M J, Anderson M G, Stolzenberg E D, Kari P U, Zasloff M, Wilson J M. Cell. 1997;88:1–9. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 3.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 4.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Jr, Ganz T. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harder J, Bartels J, Christophers E, Schroder J-M. Nature (London) 1997;387:861–862. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Wang I, Lehrer R I. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 7.McCray P B, Jr, Bentley L. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 8.Welsh M J, Boat T F, Tsui L-C, Beaudet A L. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- 9.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 10.Zabner J, Zeiher B G, Friedman E, Welsh M J. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo M, Finkbeiner W E, Widdicombe J H. Am J Physiol. 1991;263:L105–L117. [Google Scholar]
- 12.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox J N. J Histochem Cytochem. 1993;41:1725–1733. doi: 10.1177/41.12.8245419. [DOI] [PubMed] [Google Scholar]
- 14.Porter E M, Liu L, Oren A, Anton P A, Ganz T. Infect Immun. 1997;65:2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frackman S, Anhalt M, Nealson K H. J Bacteriol. 1990;172:5767–5773. doi: 10.1128/jb.172.10.5767-5773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson A B, Bohling T, Payvandi F, Rennard S I. J Lab Clin Med. 1990;115:148–158. [PubMed] [Google Scholar]
- 17.Diamond G, Jones D E, Bevins C L. Proc Natl Acad Sci USA. 1993;90:4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond G, Russell J P, Bevins C L. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolzenberg E D, Anderson G M, Ackermann M R, Whitlock R H, Zasloff M. Proc Natl Acad Sci USA. 1997;94:8686–8690. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Wang L, Jia H P, Zhao C, Heng H H Q, Schutte B C, McCray P B, Jr, Ganz T. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Zhao C, Heng H H Q, Ganz T. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 23.Wilmott R W, Kassab J T, Kilian P L, Benjamin W R, Douglas S D, Wood R E. Am Rev Respir Dis. 1990;142:365–368. doi: 10.1164/ajrccm/142.2.365. [DOI] [PubMed] [Google Scholar]
- 24.Ganz T, Lehrer R I. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer R I, Lichtenstein A K, Ganz T. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 26.Knowles M R, Robinson J M, Wood R E, Pue C A, Mentz W M, Wagner G C, Gatzy J T, Boucher R C. J Clin Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hull J, Skinner W, Robertson C, Phelan P. Am J Respir Crit Care Med. 1998;157:10–14. doi: 10.1164/ajrccm.157.1.9703045. [DOI] [PubMed] [Google Scholar]
- 28.Joris L, Dab I, Quinton P M. Am Rev Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 29.Gilljam H, Ellin A, Strandvik B. Scand J Clin Lab Invest. 1989;49:121–124. doi: 10.3109/00365518909105409. [DOI] [PubMed] [Google Scholar]
- 30.Zabner J, Smith J J, Karp P H, Widdicombe J H, Welsh M J. Mol Cell. 1998;2:397–403. doi: 10.1016/s1097-2765(00)80284-1. [DOI] [PubMed] [Google Scholar]