Abstract
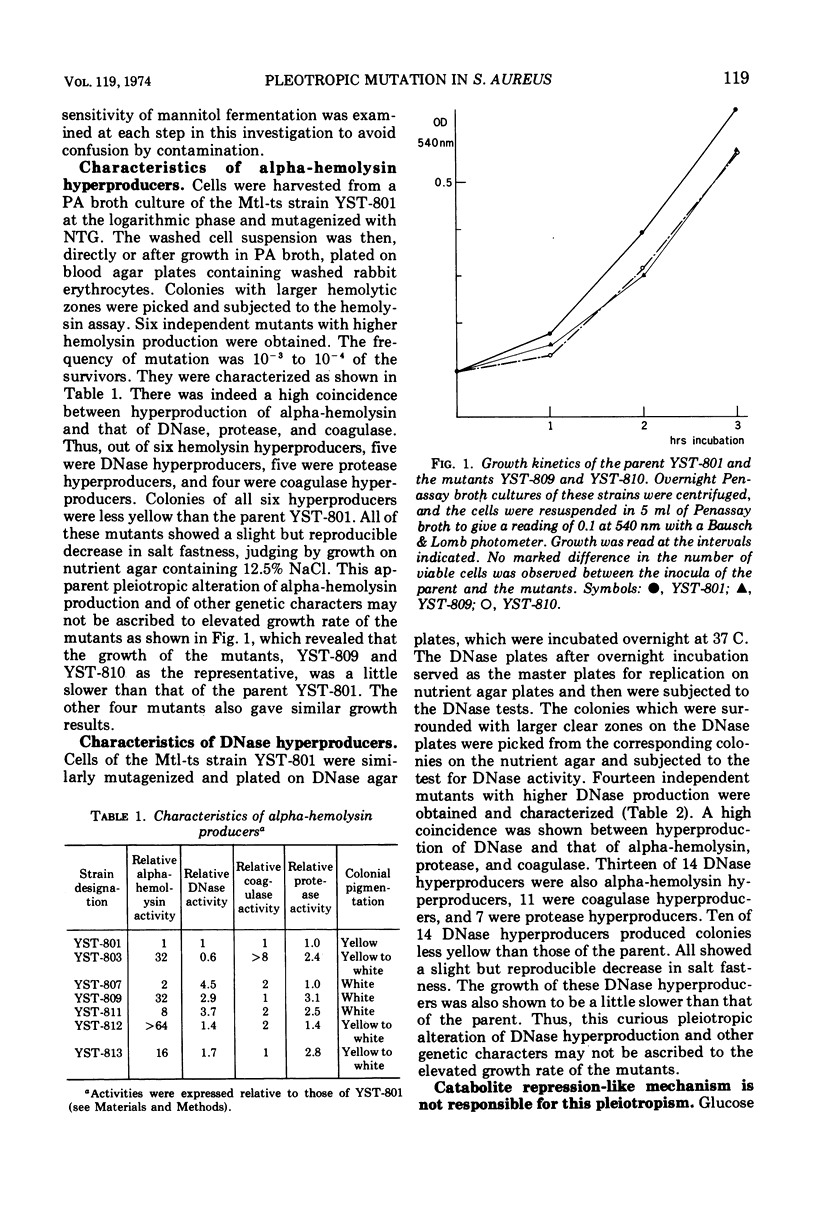
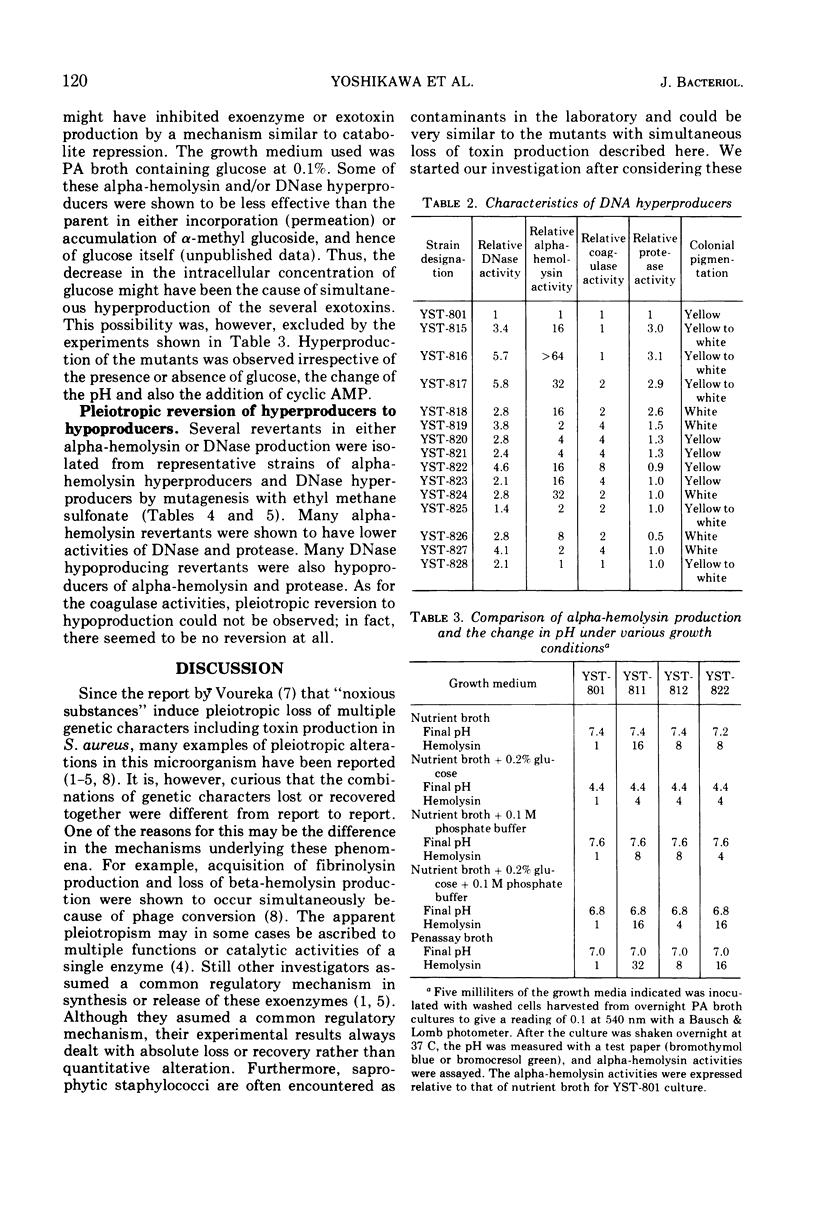
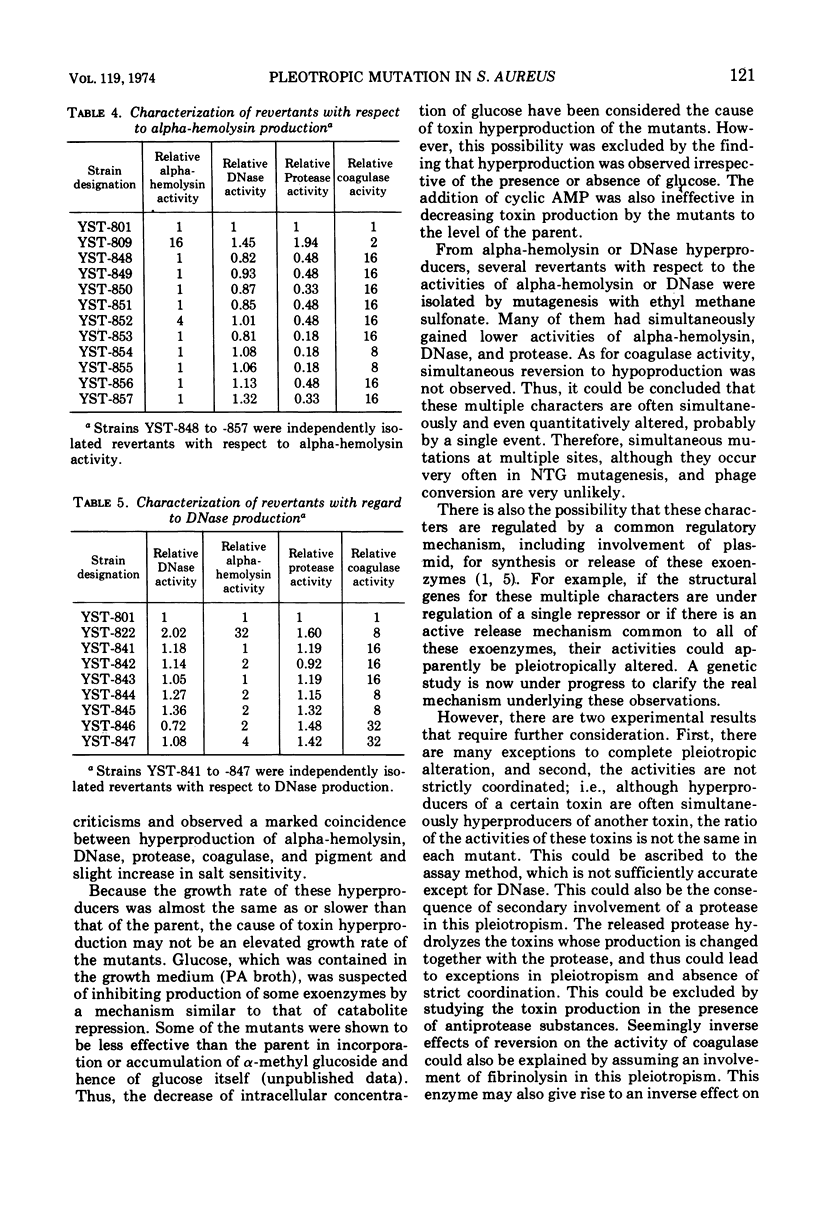
Pleiotropic alteration of several genetic characters including toxin production was quantitatively shown with a strain of Staphylococcus aureus of phage type 80, 81 which had been given a very specific genetic marker (temperature sensitivity of mannitol fermentation) to avoid confusion by contamination. Thus, alpha-hemolysin hyperproducers obtained by N-methyl-N′-nitro-N-nitrosoguanidine (NTG) mutagenesis were very often hyperproducers of DNase, coagulase, and protease. Their colonies were less yellow than the parent. DNase hyperproducers obtained after NTG mutagenesis were also often hyperproducers of alpha-hemolysin, coagulase, and protease, with colonies less yellow than the parent. Almost all of the revertants obtained by mutagenesis with ethyl methane sulfonate with respect to alpha-hemolysin or DNase were shown to have simultaneously become hypoproducers of alpha-hemolysin, DNase, and protease. Since the pleiotropic alteration of multiple functions was thus quantitatively confirmed, the mechanism underlying this phenomenon should probably be related to a regulatory mechanism common to them.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forsgren A., Nordström K., Philipson L., Sjöquist J. Protein A mutants of Staphylococcus aureus. J Bacteriol. 1971 Jul;107(1):245–250. doi: 10.1128/jb.107.1.245-250.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARMON S. A., BALDWIN J. N. NATURE OF THE DETERMINANT CONTROLLING PENICILLINASE PRODUCTION IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Mar;87:593–597. doi: 10.1128/jb.87.3.593-597.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORMAN R. Z. COAGULASE-NEGATIVE MUTANTS OF STAPHYLOCOCCUS AUREUS: GENETIC STUDIES. J Bacteriol. 1963 Sep;86:363–369. doi: 10.1128/jb.86.3.363-369.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchy J. K., Rosenblum E. D. Biological properties of alpha-toxin mutants of Staphylococcus aureus. J Bacteriol. 1966 Sep;92(3):575–579. doi: 10.1128/jb.92.3.575-579.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn G. S., Friedman J. Isolation of mutants of Staphylococcus aureus lacking extracellular nuclease activity. J Bacteriol. 1970 Mar;101(3):921–924. doi: 10.1128/jb.101.3.921-924.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. [Isolation, purification, crystallization and enzymatic chemical-properties of extracellular nuclease of Staphylococcus aureus]. Seikagaku. 1969 Jun;41(6):264–271. [PubMed] [Google Scholar]
- VOUREKA A. Induced variations in a penicillin-resistant staphylococcus. J Gen Microbiol. 1952 May;6(3-4):352–360. doi: 10.1099/00221287-6-3-4-352. [DOI] [PubMed] [Google Scholar]
- Winkler K. C., de Waart J., Grootsen C. Lysogenic conversion of staphylococci to loss of beta-toxin. J Gen Microbiol. 1965 Jun;39(3):321–333. doi: 10.1099/00221287-39-3-321. [DOI] [PubMed] [Google Scholar]



