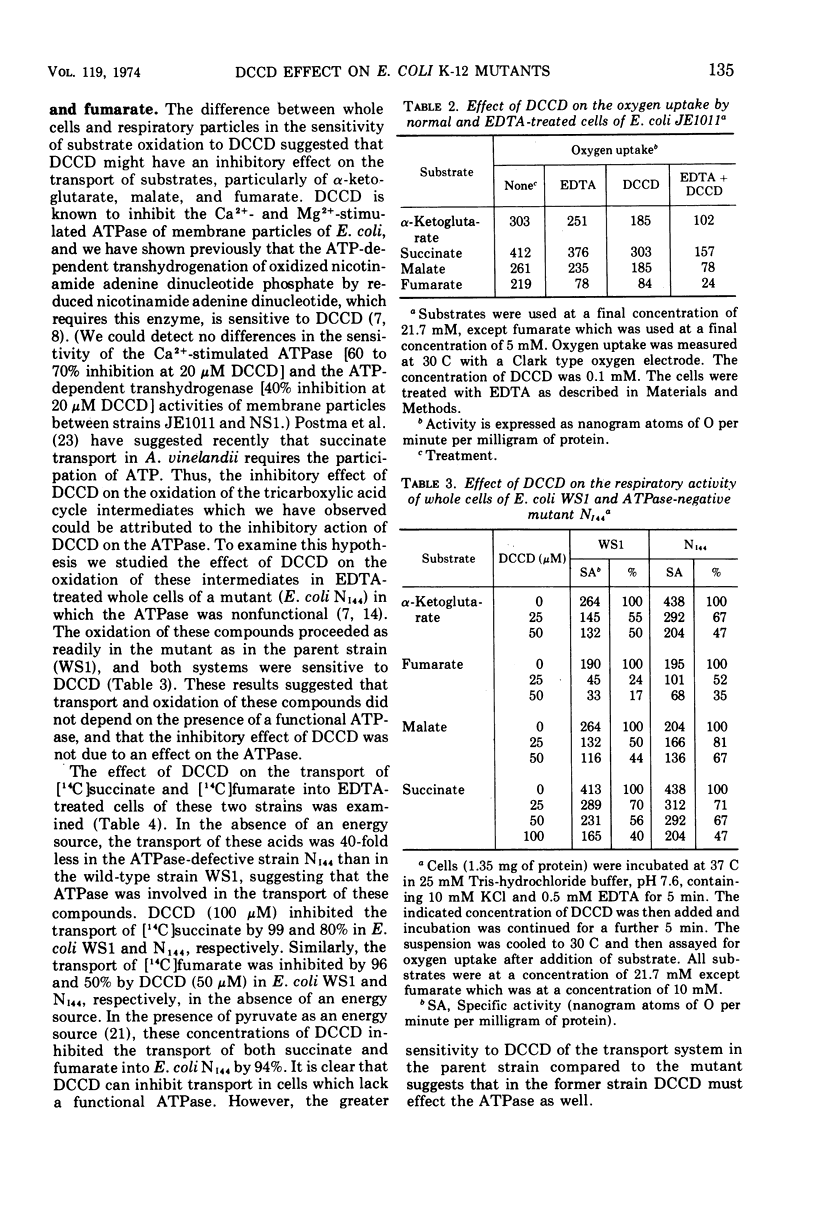
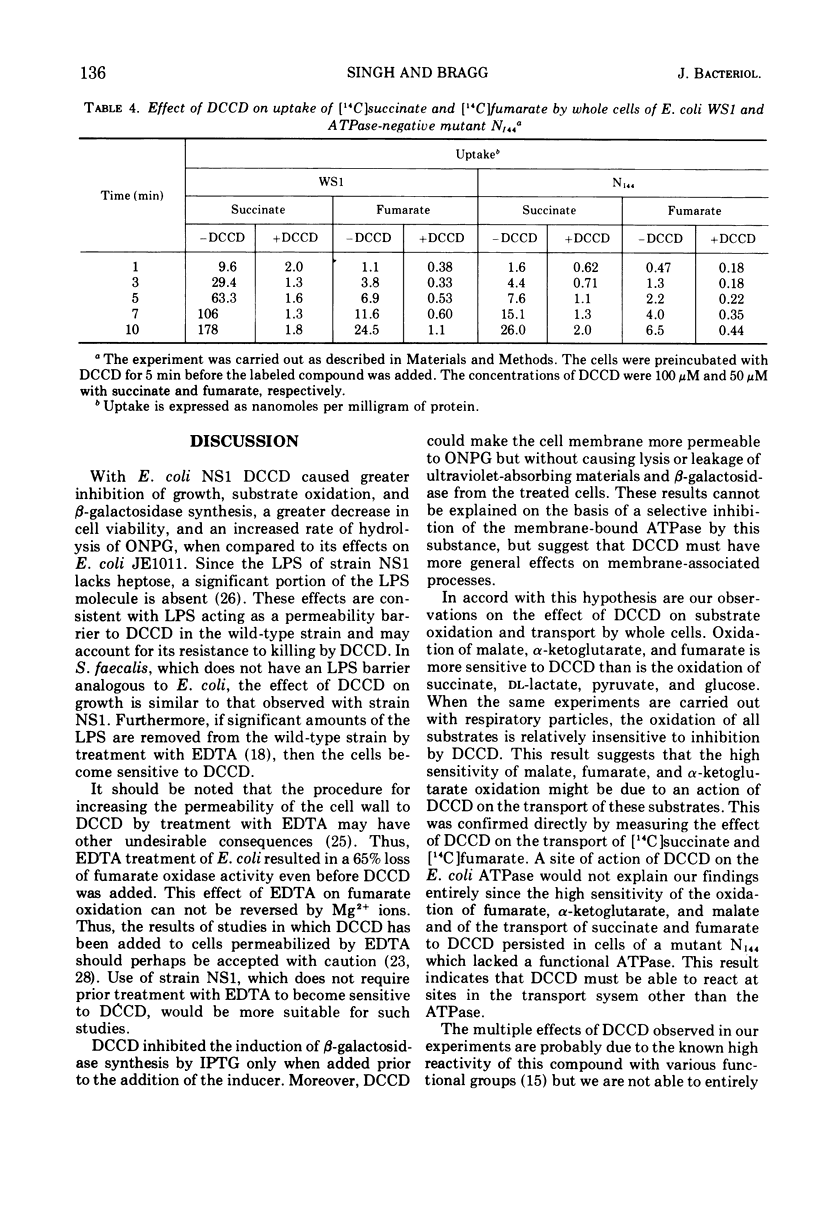
Abstract
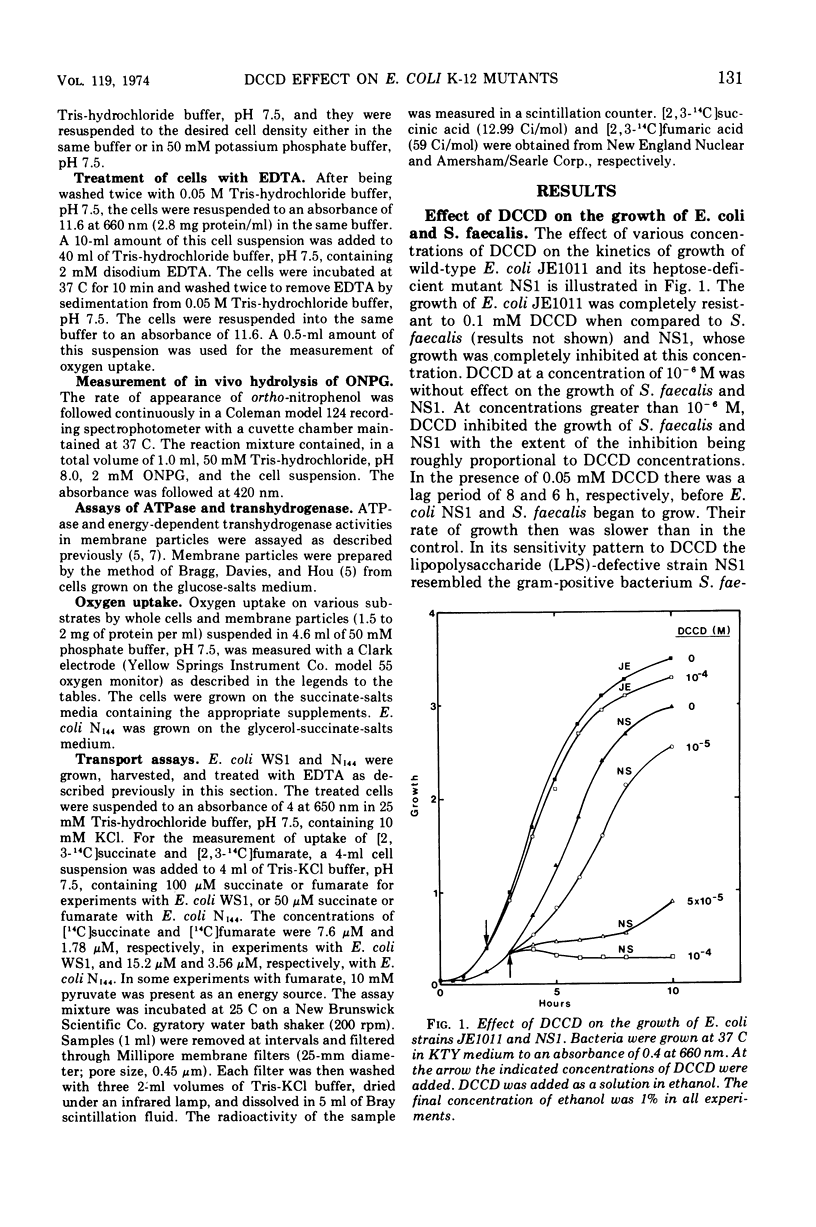
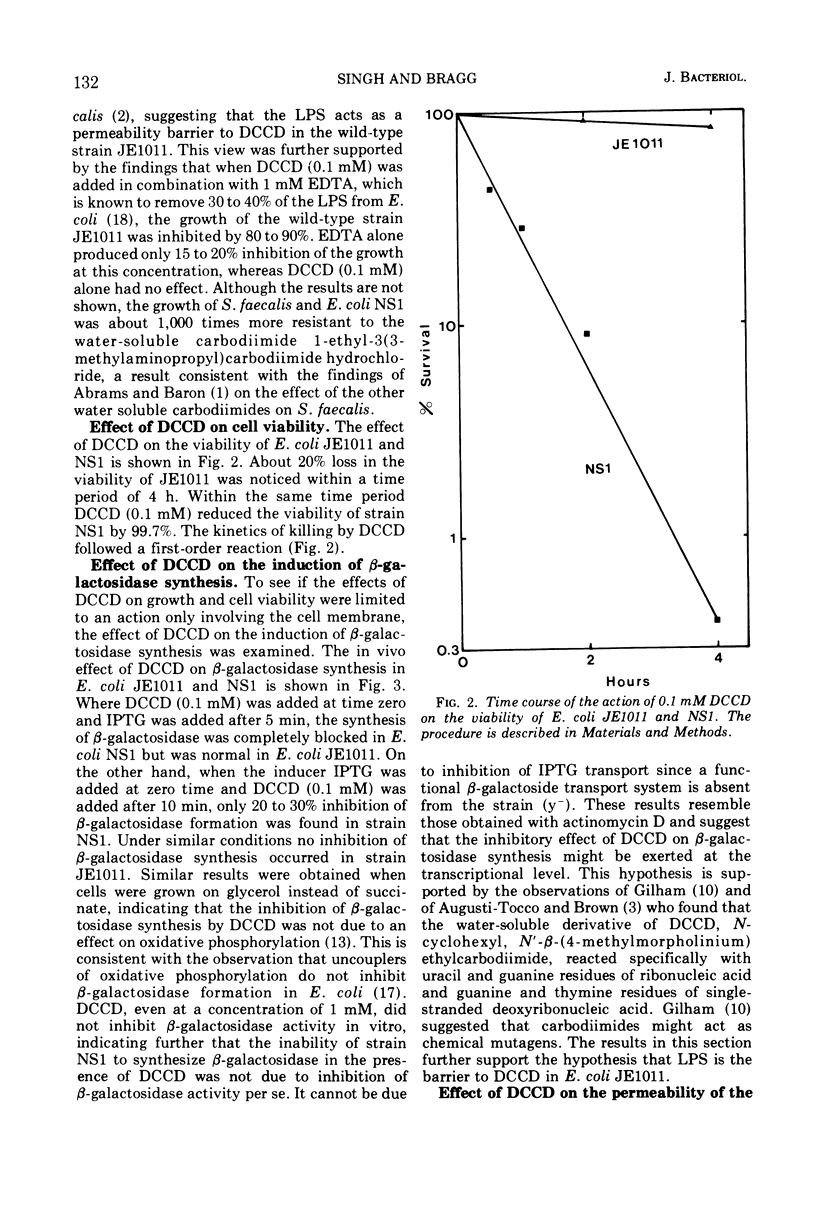
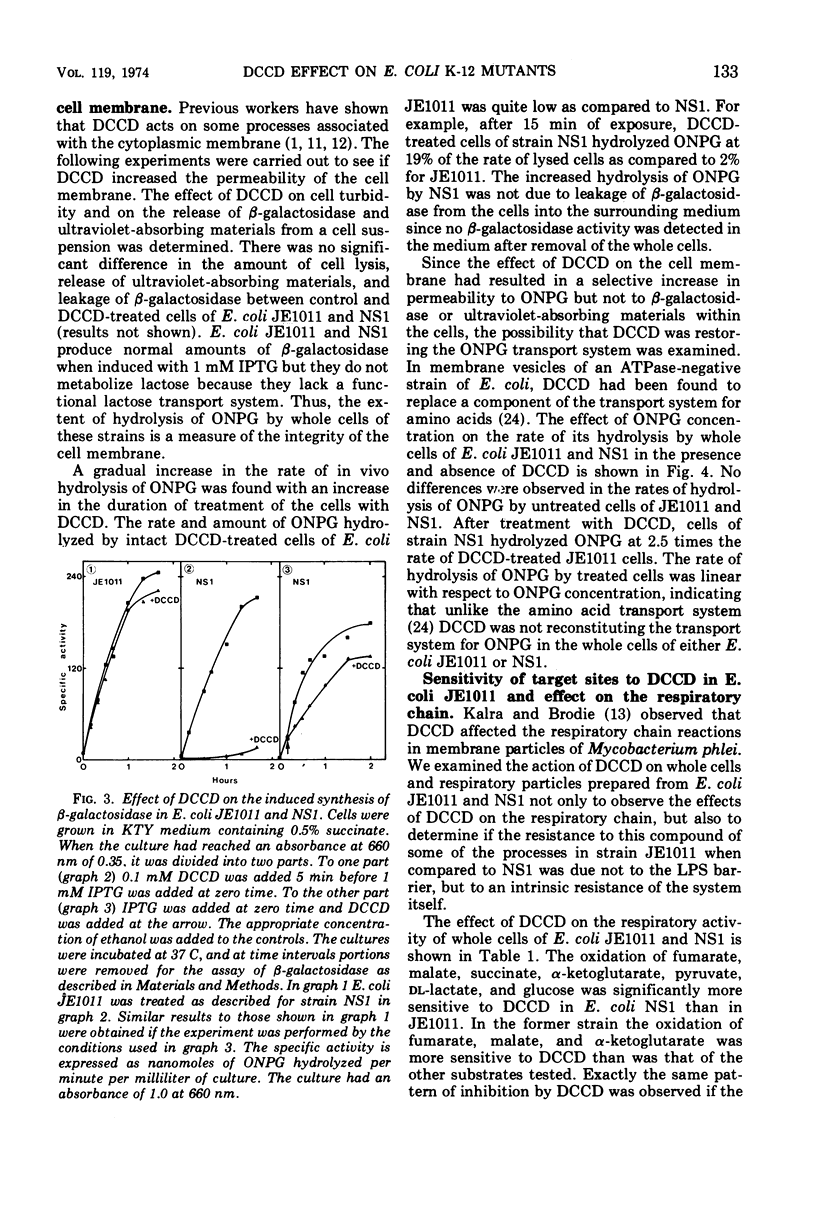
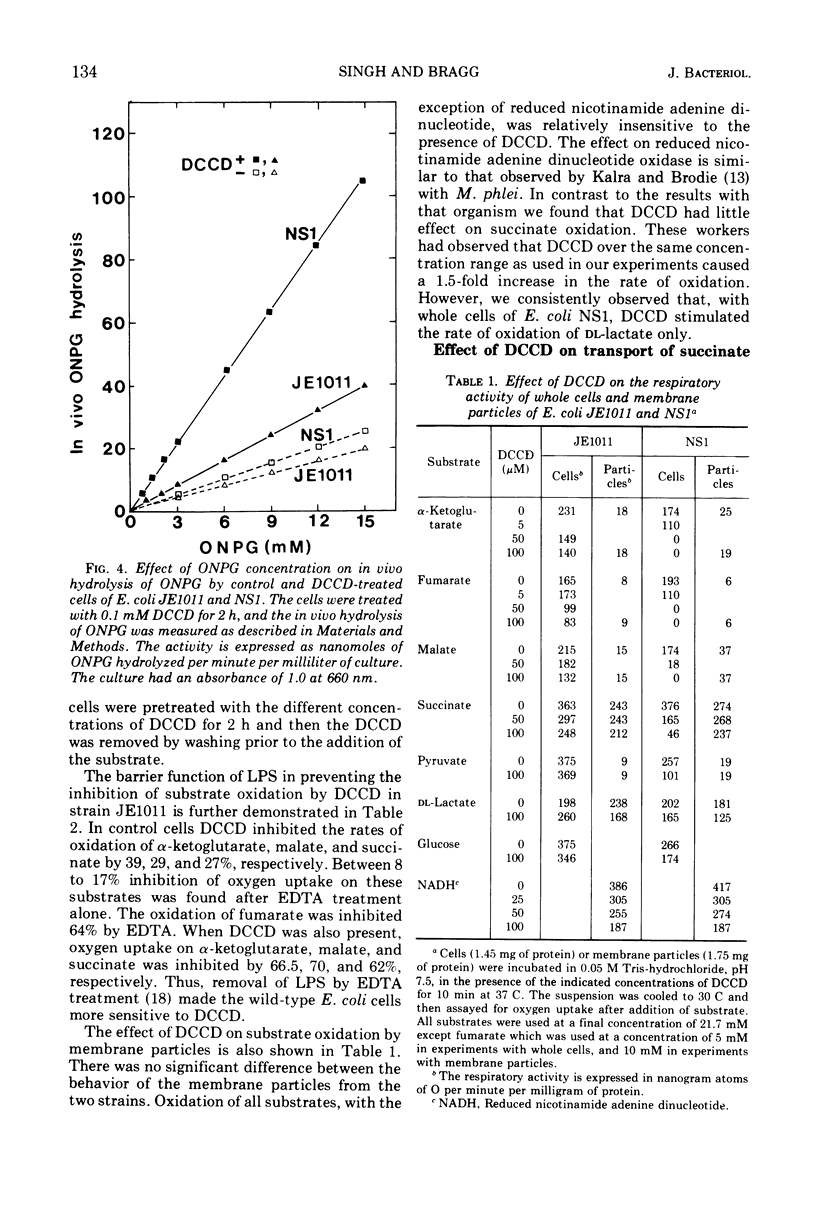
The effects of N,N′-dicyclohexylcarbodiimide (DCCD) on the growth of Streptococcus faecalis, and on the growth, β-galactosidase synthesis, and various membrane-mediated processes, were studied in wild-type Escherichia coli JE1011 and its lipopolysaccharide-defective mutant NS1. DCCD (0.1 mM) completely inhibited the growth of S. faecalis and E. coli NS1 but had little effect on strain JE1011. The same amount of DCCD with E. coli NS1, but not with E. coli JE1011, inhibited the induction of β-galactosidase, increased the permeability of the cells to o-nitrophenyl-β-d-galactoside without causing extensive cell lysis or release of ultraviolet-absorbing materials, and inhibited the oxidation of certain intermediates of the tricarboxylic acid cycle. Inhibition of the oxidation of malate, fumarate, and α-ketoglutarate by DCCD appeared to be at the level of the transport system for these compounds. Inhibition of the membrane-bound adenosine triphosphatase by DCCD was not entirely responsible for these effects, since oxidation of these substances, and transport of [14C]succinate and [14C]fumarate, was inhibited by DCCD in a mutant, N144, which lacked adenosine triphosphatase activity. It is concluded that lipopolysaccharide forms a barrier to DCCD in wild-type E. coli, and that DCCD can inhibit several processes in the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Baron C. Inhibitory action of carbodiimides on bacterial membrane ATPase. Biochem Biophys Res Commun. 1970 Nov 25;41(4):858–863. doi: 10.1016/0006-291x(70)90162-2. [DOI] [PubMed] [Google Scholar]
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Augusti-Tocco G., Brown G. L. Reaction of N-cyclohexyl, N'-beta (4-methylmorpholinium) ethyl carbodiimide iodide with nucleic acids and polynucleotides. Nature. 1965 May 15;206(985):683–685. doi: 10.1038/206683a0. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Davies P. L., Hou C. Function of energy-dependent transhydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1248–1255. doi: 10.1016/0006-291x(72)90969-2. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Purification of a factor for both aerobic-driven and ATP-driven energy-dependent transhydrogenases of Escherichia coli. FEBS Lett. 1972 Dec 15;28(3):309–312. doi: 10.1016/0014-5793(72)80738-5. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reconstitution of energy-dependent transhydrogenase in ATPase-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Feb 5;50(3):729–736. doi: 10.1016/0006-291x(73)91305-3. [DOI] [PubMed] [Google Scholar]
- Evans D. J. Membrane Mg-(Ca)-Activated Adenosine Triphosphatase of Escherichia coli: Characterization in the Membrane-Bound and Solubilized States. J Bacteriol. 1970 Dec;104(3):1203–1212. doi: 10.1128/jb.104.3.1203-1212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Dio 9 and chlorhexidine: inhibitors of membrane-bound ATPase and of cation transport in Streptococcus faecalis. Biochim Biophys Acta. 1969 Jun 3;183(1):129–136. doi: 10.1016/0005-2736(69)90136-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Kovác L., Kuzela S. Effect of uncoupling agents and azide on the synthesis of beta-galactosidase in aerobically and anaerobically grown Escherichia coli. Biochim Biophys Acta. 1966 Oct 31;127(2):355–365. [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Postma P. W., Cools A., van Dam K. The transport of Krebs-cycle intermediates in Azotobacter vinelandii under various metabolic conditions. Biochim Biophys Acta. 1973 Aug 9;318(1):91–104. doi: 10.1016/0005-2736(73)90339-8. [DOI] [PubMed] [Google Scholar]
- Prezioso G., Hong J. S., Kerwar G. K., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XII. Active transport by a mutant of Escherichia coli uncoupled for oxidative phosphorylation. Arch Biochem Biophys. 1973 Feb;154(2):575–582. doi: 10.1016/0003-9861(73)90011-8. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Cheng K. J., Costerton J. W., Idziak E. S., Ingram J. M. Sensitivity of normal and mutant strains of Escherichia coli to actinomycin-D. Can J Microbiol. 1972 Jun;18(6):909–915. doi: 10.1139/m72-139. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



